Novel Aptamers Targeting Sclerostin Loop3 Improve Skeletal and Muscle Properties Without Adverse Cardiovascular Effects in Orchiectomized Mice
- PMID: 40464222
- PMCID: PMC12134771
- DOI: 10.1002/jcsm.13831
Novel Aptamers Targeting Sclerostin Loop3 Improve Skeletal and Muscle Properties Without Adverse Cardiovascular Effects in Orchiectomized Mice
Abstract
Background: The Wnt/β-catenin pathway and its bone-specific inhibitor, sclerostin, play important roles in skeletal development and homeostasis. The humanized sclerostin antibody, romosozumab, can significantly increase bone mineral density (BMD) of patients with osteoporosis, but it may also increase cardiovascular adverse events, particularly in male patients. We try to investigate the effects of novel aptamers targeting the sclerostin loop3 on the skeleton and muscle of orchiectomized (ORX) mice.
Methods: After 12 weeks of ORX surgery, mice were randomly assigned to receive treatment with sclerostin aptamers (Apc001OA or Apc001OA-d6), alendronate (ALN), teriparatide (PTH 1-34) or phosphate-buffered saline (PBS). After 12 weeks of treatment, skeletal and muscle properties and safety indicators were evaluated in detail.
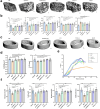
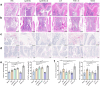
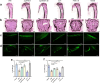
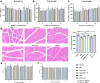
Results: Treatment with Apc001OA and Apc001OA-d6 significantly increased trabecular BMD at the femur by +11.9% and +17.1%, improved parameters of bone microarchitecture (BV/TV by +84.5% and +106.8%), bone strength (maximum load by +30.5% and +31.6%) and bone histological properties (all p < 0.05 vs. PBS group). The therapeutic effects were similar among Apc001OA, Apc001OA-d6, ALN and PTH 1-34 groups (all p > 0.05). After treatment with Apc001OA or Apc001OA-d6, serum sclerostin levels significantly decreased by 25.0% and 24.9% (p < 0.05 vs. PBS group). The expression levels of key genes in the Wnt/β-catenin pathway, Ctnnb1 and Lef1 significantly increased by 2.4- and 3.4-fold in the Apc001OA group and by 2.5- and 3.5-fold in the Apc001OA-d6 group (p < 0.05 vs. PBS group), indicating that the aptamers improved bone properties through activating Wnt/β-catenin pathway. Apc001OA and Apc001OA-d6 significantly improved rotarod latency (p < 0.05 vs. PBS group) of ORX mice, and Apc001OA-d6 could increase forelimb grip strength. Apc001OA, Apc001OA-d6 and PTH 1-34 improved histological properties of muscle in ORX mice. No lesions or pathological changes were observed in the heart, aortic roots, liver, spleen, lungs or kidneys. Immunohistochemistry revealed no abnormal staining of interleukin 6 (IL-6) and tumour necrosis factor-α (TNF-α) in the heart. There was no significant difference in serum concentrations of cardiac functional biomarkers, including creatine kinase-MB (CK-MB), cardiac troponin I (cTnI), B-type natriuretic peptide (BNP) and inflammatory mediators (IL-6 and TNF-α) across all groups, indicating that Apc001OA and Apc001OA-d6 had no adverse cardiovascular effects in ORX mice.
Conclusions: The novel aptamers Apc001OA and Apc001OA-d6, targeting sclerostin loop3, could significantly increase BMD and improve bone microarchitecture, bone biomechanics, muscle function and histological properties of muscle and bone in ORX mice, without adverse cardiovascular effects. These aptamers may serve as potential agents for treating osteoporosis and sarcopenia in men.
Keywords: adverse cardiovascular effects; aptamers; male osteoporosis; sarcopenia; sclerostin loop3.
© 2025 The Author(s). Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Ensrud K. E. and Crandall C. J., “Osteoporosis,” Annals of Internal Medicine 167 (2017): Itc17–itc32. - PubMed
-
- Vilaca T., Eastell R., and Schini M., “Osteoporosis in Men,” Lancet Diabetes & Endocrinology 10 (2022): 273–283. - PubMed
-
- Sing C. W., Lin T. C., Bartholomew S., et al., “Global Epidemiology of Hip Fractures: Secular Trends in Incidence Rate, Post‐Fracture Treatment, and All‐Cause Mortality,” Journal of Bone and Mineral Research: The Official Journal of the American Society for Bone and Mineral Research 38 (2023): 1064–1075. - PubMed
-
- Marini F., Giusti F., Palmini G., and Brandi M. L., “Role of Wnt Signaling and Sclerostin in Bone and as Therapeutic Targets in Skeletal Disorders,” Osteoporosis International 34 (2023): 213–238. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

