Crucial roles of intracellular cyclic di-GMP in impacting the genes important for extracellular electron transfer by Geobacter metallireducens
- PMID: 40464557
- PMCID: PMC12285248
- DOI: 10.1128/aem.00727-25
Crucial roles of intracellular cyclic di-GMP in impacting the genes important for extracellular electron transfer by Geobacter metallireducens
Abstract
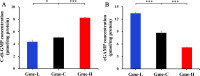
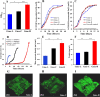
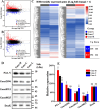
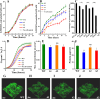
To investigate the roles of intracellular c-di-GMP in bacterial extracellular electron transfer (EET), three Geobacter metallireducens strains with high (Gme-H), intermediate (Gme-C), and low (Gme-L) intracellular levels of c-di-GMP were constructed via the synthetic biology approach. Compared to Gme-C, Gme-H showed similar Fe(III) reduction rates, formed thicker biofilms on conductive and nonconductive surfaces, and produced more electricity, but showed delayed ability for electricity production. Gme-L formed thinner biofilms on nonconductive surfaces and reduced Fe(III)-citrate faster, but showed slower reduction of ferrihydrite in comparison to Gme-C. Although it produced electricity much faster, Gme-L produced less electricity and formed slightly less amounts of biofilms on anodes, as compared to Gme-C. The mRNA levels of multiple genes encoding c-type cytochromes (c-Cyts) and extracellular pilin protein PilA-N were differentially regulated in Gme-L or Gme-H in comparison to that in Gme-C. Expressions of the genes for PilA-N and extracellular c-Cyt Gmet2896 were increased by high c-di-GMP. Low c-di-GMP increased the gene expressions of the porin-cytochromes in the outer membrane. Further investigation also identified new c-di-GMP-regulated genes directly involved in the EET of G. metallireducens, such as those for the c-Cyts of extracellular Gmet0601, the periplasmic Gmet1703 and Gmet1809 on the cytoplasmic membrane, as deletions of these genes impaired bacterial reductions of extracellular ferrihydrite and anode. Thus, intracellular c-di-GMP impacted multiple genes of G. metallireducens whose protein products might transfer electrons from the cytoplasmic membrane, through the periplasm, across the outer membrane to and in the extracellular environment.IMPORTANCEBis-(3'-5')-cyclic dimeric guanosine monophosphate (c-di-GMP) is ubiquitous in bacterial cells where it regulates a variety of bacterial processes, which range from biofilm formation, bacterial virulence to cell cycle progression. However, its role in regulating bacterial extracellular electron transfer is much less characterized. This investigation shows the crucial roles of intracellular c-di-GMP in impacting the extracellular electron transfer of the Gram-negative bacterium Geobacter metallireducens. The gene expressions of the multiheme c-type cytochromes in the bacterial cytoplasmic membrane, periplasm, outer membrane, and extracellular environment, as well as the gene expression of extracellular pilin protein PilA-N, are all impacted by c-di-GMP. Although how it impacts the expression of these genes is currently unclear, c-di-GMP affects the entire extracellular electron transfer process of G. metallireducens from the cytoplasmic membrane, through the periplasm and across the outer membrane to and in the extracellular environment.
Keywords: Fe(III)-reduction; Geobacter metallireducens; biofilms; cyclic di-GMP; extracellular electron transfer; microbial fuel cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
MeSH terms
Substances
Grants and funding
- 2018YFA0901303/National Key Research and Development Program of China
- 91851211, 42272353, 42277065, 42202340/National Natural Science Foundation of China
- 122-G1323522144, 122-162301202678/Fundamental Research Funds for Central Universities of Chinese Central Government, China University of Geosciences-Wuhan
LinkOut - more resources
Full Text Sources

