Donor-derived microbial engraftment and gut microbiota shifts associated with weight loss following fecal microbiota transplantation
- PMID: 40464558
- PMCID: PMC12285249
- DOI: 10.1128/aem.00120-25
Donor-derived microbial engraftment and gut microbiota shifts associated with weight loss following fecal microbiota transplantation
Abstract
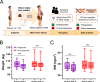
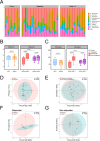
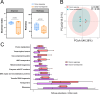
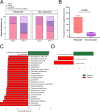
Fecal microbiota transplantation (FMT) is a promising treatment for microbiota dysbiosis and may provide metabolic benefits for obesity. However, its mechanisms and variability in clinical outcomes remain poorly understood. This 12-week multicenter, single-arm study evaluated the efficacy of FMT for weight loss and explored the role of donor-derived microbial engraftment and functional shifts in mediating weight loss among overweight and obese individuals. Twenty-three participants (body mass index ≥24 kg/m²) without diabetes received three biweekly FMT sessions via a nasojejunal tube. Fecal samples from participants and donors were analyzed using metagenomic sequencing. By week 12, 52% of participants were classified as responders, achieving significant weight loss of ≥5% from baseline, with an average weight loss of 7.98 ± 2.69 kg (P < 0.001). In contrast, non-responders lost 2.90 ± 1.89 kg (P < 0.001). Responders exhibited a significantly higher proportion of donor-derived microbial strains post-FMT compared to non-responders (37.8% vs 15.2%, P = 0.020). Notably, key taxa, including Phascolarctobacterium (P = 0.034) and Acidaminococcaceae (P = 0.012), increased significantly in abundance in responders post-FMT, indicating successful microbial engraftment as a critical determinant of therapeutic success. These findings suggest that FMT is a viable intervention for weight loss in obese individuals. Successful donor-derived microbial engraftment strongly correlates with weight loss efficacy, highlighting the potential of microbiota-targeted therapies in obesity management and providing insights into the mechanisms underlying FMT outcomes.IMPORTANCEPrior research indicates that fecal microbiota transplantation (FMT) is a promising treatment for diseases related to microbiota imbalance, potentially providing metabolic benefits for obesity. However, the specific role of donor-derived microbial engraftment in driving clinical efficacy has remained unclear. In this study, we evaluated the efficacy of FMT in promoting weight loss and explored the role of donor-derived bacterial strains in this process. Our findings demonstrate that the successful engraftment of specific donor-derived taxa, such as Phascolarctobacterium and Acidaminococcaceae, is strongly associated with significant weight loss. This highlights the critical interplay between donor microbiota and recipient gut environment. These findings underscore the potential of microbiota-targeted therapies as a novel strategy for obesity management.CLINICAL TRIALSThis study is registered with the Chinese Clinical Trial Registry as ChiCTR1900024760.
Keywords: fecal microbiota transplantation; gut microbiome; metagenome; obesity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Fecal microbiota transplantation for the treatment of recurrent Clostridioides difficile (Clostridium difficile).Cochrane Database Syst Rev. 2023 Apr 25;4(4):CD013871. doi: 10.1002/14651858.CD013871.pub2. Cochrane Database Syst Rev. 2023. PMID: 37096495 Free PMC article.
-
Intestinal inflammation and microbiota modulation impact cochlear function: emerging insights in gut-ear axis.Cell Commun Signal. 2025 Jul 26;23(1):357. doi: 10.1186/s12964-025-02338-1. Cell Commun Signal. 2025. PMID: 40713718 Free PMC article.
-
Gut microbiome-based interventions for the management of obesity in children and adolescents aged up to 19 years.Cochrane Database Syst Rev. 2025 Jul 10;7(7):CD015875. doi: 10.1002/14651858.CD015875. Cochrane Database Syst Rev. 2025. PMID: 40637175 Review.
-
Variability of strain engraftment and predictability of microbiome composition after fecal microbiota transplantation across different diseases.Nat Med. 2022 Sep;28(9):1913-1923. doi: 10.1038/s41591-022-01964-3. Epub 2022 Sep 15. Nat Med. 2022. PMID: 36109637 Free PMC article.
-
Host origin of microbiota drives functional recovery and Clostridioides difficile clearance in mice.mBio. 2025 Jul 9;16(7):e0110825. doi: 10.1128/mbio.01108-25. Epub 2025 Jun 2. mBio. 2025. PMID: 40454811 Free PMC article.
References
-
- 2023. World obesity atlas 2023
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

