SARS-CoV-2 infection enhancement by amphotericin B: implications for disease management
- PMID: 40464579
- PMCID: PMC12282131
- DOI: 10.1128/jvi.00519-25
SARS-CoV-2 infection enhancement by amphotericin B: implications for disease management
Abstract
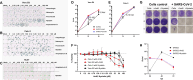
Severe coronavirus disease 2019 (COVID-19) patients who require hospitalization are at high risk of invasive pulmonary mucormycosis. Amphotericin B (AmB), which is the first-line therapy for invasive pulmonary mucormycosis, has been shown to promote or inhibit replication of a spectrum of viruses. In this study, we first predicted that AmB and nystatin had strong interactions with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) proteins using in silico screening, indicative of drugs with potential therapeutic activity against this virus. Subsequently, we investigated the impact of AmB, nystatin, natamycin, fluconazole, and caspofungin on SARS-CoV-2 infection and replication in vitro. Results showed that AmB and nystatin actually increased SARS-CoV-2 replication in Vero E6, Calu-3, and Huh7 cells. At optimal concentrations, AmB and nystatin increase SARS-CoV-2 replication by up to 100- and 10-fold in Vero E6 and Calu-3 cells, respectively. The other antifungals tested had no impact on SARS-CoV-2 infection in vitro. Drug kinetic studies indicate that AmB enhances SARS-CoV-2 infection by promoting viral entry into cells. Additionally, knockdown of genes encoding for interferon-induced transmembrane (IFITM) proteins 1, 2, and 3 suggests AmB enhances SARS-CoV-2 cell entry by overcoming the antiviral effect of the IFITM3 protein. This study further elucidates the role of IFITM3 in viral entry and highlights the potential dangers of treating COVID-19 patients, with invasive pulmonary mucormycosis, using AmB.IMPORTANCEAmB and nystatin are common treatments for fungal infections but were predicted to strongly interact with SARS-CoV-2 proteins, indicating their potential modulation or inhibition against the virus. However, our tests revealed that these antifungals, in fact, enhance SARS-CoV-2 infection by facilitating viral entry into cells. The magnitude of enhancement could be up to 10- or 100-fold, depending on cell lines used. These findings indicate that AmB and nystatin have the potential to enhance disease when given to patients infected with SARS-CoV-2 and therefore should not be used for treatment of fungal infections in active COVID-19 cases.
Keywords: SARS-CoV-2; amphotericin B; disease management; infection enhancement.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

