Interferon-stimulated gene MCL1 inhibits foot-and-mouth disease virus replication by modulating mitochondrial dynamics and autophagy
- PMID: 40464580
- PMCID: PMC12282159
- DOI: 10.1128/jvi.00581-25
Interferon-stimulated gene MCL1 inhibits foot-and-mouth disease virus replication by modulating mitochondrial dynamics and autophagy
Abstract
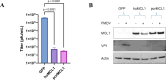
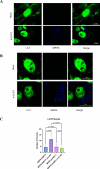
Interferons (IFNs) and the IFN-stimulated genes (ISGs) that they induce are effective in reducing the replication of foot and mouth disease virus (FMDV). The use of a high-throughput ISG screen identified the ISG myeloid cell leukemia 1 (MCL1) as an ISG with an antiviral effect against an FMDV replicon system. In this study, we demonstrated that overexpression of MCL1 inhibits FMDV replication by reducing approximately 4 logs of virus titers in porcine cells. We then explored the regulatory pathways associated with MCL1 to determine the specific antiviral mechanisms against FMDV. Our findings indicated that the antiviral mechanism does not involve apoptosis regulation or alterations in cell cycle phase heterogeneity. Analysis of mitochondrial function, through measurement of mitochondrial oxygen consumption rate, demonstrated that overexpression of MCL1 results in increased mitochondrial respiration and ATP production, whereas FMDV infection reduces both processes. Moreover, MCL1 overexpression resulted in elongated mitochondrial morphology, contrasting with the fragmented and punctate morphology observed during FMDV infection. Importantly, these changes in mitochondrial dynamics were independent of MCL1's regulation of mitochondrial calcium flux. We also found that MCL1 overexpression suppresses autophagy, which is known to be necessary for FMDV replication. Our data indicate that MCL1 is a potent antiviral ISG against FMDV and highlight the importance of mitochondrial dynamics and autophagy in FMDV replication.IMPORTANCEIn this study, we have successfully used a high-throughput ISG screening approach to measure the inhibition of FMDV replication using an RNA replicon system for the first time. This screen led to the identification of the potent antiviral effects of a relatively lesser-known ISG called MCL1. Our findings reveal that MCL1 exerts its antiviral functions through the regulation of mitochondrial dynamics and autophagy. Although mitochondrial dynamics are involved in apoptosis, metabolism, redox homeostasis, stress responses, and antiviral signaling, this pathway has not been thoroughly explored in the context of FMDV infection. Further investigation into mitochondrial dynamics may facilitate the development of improved biotherapeutics for FMDV. Additionally, our studies highlight the significance of autophagy, a pathway that is needed by FMDV for replication. Ultimately, a deep understanding of all mechanisms exploited by FMDV may allow for the rational design of novel therapeutics and vaccines to control FMD.
Keywords: antiviral agents; autophagy; foot-and-mouth disease virus; interferon-stimulated genes; interferons; mitochondrial metabolism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- World Organisation for Animal Health . 2022. Foot and mouth disease. WOAH - World Organisation for Animal Health. Available from: https://www.woah.org/en/disease/foot-and-mouth-disease
-
- World Organisation for Animal Health . 2022. Terrestrial code online access. WOAH - World Organisation for Animal Health. Available from: https://www.woah.org/en/what-we-do/standards/codes-and-manuals/
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

