Prevention of atrial fibrillation with SGLT2 inhibitors across the spectrum of cardiovascular disorders: a meta-analysis of randomized controlled trials
- PMID: 40464647
- PMCID: PMC12342982
- DOI: 10.1093/ehjcvp/pvaf040
Prevention of atrial fibrillation with SGLT2 inhibitors across the spectrum of cardiovascular disorders: a meta-analysis of randomized controlled trials
Abstract
Aims: The ability of sodium-glucose co-transporter 2 (SGLT2) inhibitors to prevent atrial fibrillation (AF) has been evaluated in various studies with conflicting results. This study aimed to determine whether SGLT2 inhibitors have a protective effect against AF depending on the baseline clinical condition in which the randomized controlled trials (RCTs) were conducted.
Methods and results: A trial-level meta-analysis was performed including 52 RCTs (112 031 patients) comparing SGLT2 inhibitors with placebo and reporting the number of patients who developed AF in each arm. Risk ratios (RRs) for AF development with 95% confidence intervals (95% CIs) were pooled using a random-effects model. Subgroup analyses were performed by classifying RCTs according to the inclusion criteria of each trial [diabetes, chronic kidney disease, heart failure with reduced ejection fraction (HFrEF), mildly reduced EF (HFmrEF), and preserved EF (HFpEF)]. Overall, SGLT2 inhibitors prevented AF (RR = 0.86, 95% CI 0.77-0.96). In the subgroup analysis, the AF-preventive ability of SGLT2 inhibitors was influenced by HF, being preserved in RCTs recruiting 9141 patients with HFrEF, but not in those recruiting 12 877 subjects with HFmrEF/HFpEF (P-value for group difference = 0.01). Meta-regression showed a reduced efficacy of SGLT2 inhibitors in preventing AF when more patients with hypertension or higher EF were enrolled (P < 0.01 for both).
Conclusion: Sodium-glucose co-transporter 2 inhibitors prevent AF. Their protective effect was confirmed in the HFrEF subgroup, but not in RCTs recruiting patients with HFmrEF/HFpEF, possibly indicating a different pathophysiology leading to AF among these conditions. However, given the limitations of a trial-level analysis, these findings are exploratory, pending confirmation from patient-level data.
Keywords: Atrial fibrillation; Diastolic dysfunction; Heart failure; Heart failure with preserved ejection fraction; Heart failure with reduced ejection fraction; SGLT2 inhibitors.
© The Author(s) 2025. Published by Oxford University Press on behalf of European Society of Cardiology.
Conflict of interest statement
Conflict of interest: none declared.
Figures
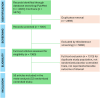



References
-
- Mebazaa A, Davison B, Chioncel O, Cohen-Solal A, Diaz R, Filippatos G, Metra M, Ponikowski P, Sliwa K, Voors AA, Edwards C, Novosadova M, Takagi K, Damasceno A, Saidu H, Gayat E, Pang PS, Celutkiene J, Cotter G. Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): a multinational, open-label, randomised, trial. Lancet 2022;400:1938–1952. - PubMed
-
- von Lewinski D, Kolesnik E, Tripolt NJ, Pferschy PN, Benedikt M, Wallner M, Alber H, Berger R, Lichtenauer M, Saely CH, Moertl D, Auersperg P, Reiter C, Rieder T, Siller-Matula JM, Gager GM, Hasun M, Weidinger F, Pieber TR, Zechner PM, Herrmann M, Zirlik A, Holman RR, Oulhaj A, Sourij H. Empagliflozin in acute myocardial infarction: the EMMY trial. Eur Heart J 2022;43:4421–4432. - PMC - PubMed
-
- Paolisso P, Belmonte M, Gallinoro E, Scarsini R, Bergamaschi L, Portolan L, Armillotta M, Esposito G, Moscarella E, Benfari G, Montalto C, Shumkova M, de Oliveira EK, Angeli F, Orzalkiewicz M, Fabroni M, Baydaroglu N, Munafò AR, D’Atri DO, Casenghi M, Scisciola L, Barbieri M, Marfella R, Gragnano F, Conte E, Pellegrini D, Ielasi A, Andreini D, Penicka M, Oreglia JA, Calabrò P, Bartorelli A, Pizzi C, Palmerini T, Vanderheyden M, Saia F, Ribichini F, Barbato E. SGLT2-inhibitors in diabetic patients with severe aortic stenosis and cardiac damage undergoing transcatheter aortic valve implantation (TAVI). Cardiovasc Diabetol 2024;23:420. - PMC - PubMed
-
- Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou F-F, Langkilde A-M, Wheeler DC. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436–1446. - PubMed
-
- Paolisso P, Bergamaschi L, Gragnano F, Gallinoro E, Cesaro A, Sardu C, Mileva N, Foà A, Armillotta M, Sansonetti A, Amicone S, Impellizzeri A, Esposito G, Morici N, Andrea OJ, Casella G, Mauro C, Vassilev D, Galie N, Santulli G, Marfella R, Calabrò P, Pizzi C, Barbato E. Outcomes in diabetic patients treated with SGLT2-inhibitors with acute myocardial infarction undergoing PCI: the SGLT2-I AMI PROTECT registry. Pharmacol Res 2023;187:106597. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

