The reaction mechanism for glycolysis side product degradation by Parkinson's disease-linked DJ-1
- PMID: 40464736
- PMCID: PMC12135873
- DOI: 10.1083/jcb.202411078
The reaction mechanism for glycolysis side product degradation by Parkinson's disease-linked DJ-1
Abstract
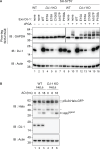
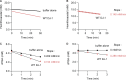
DJ-1/PARK7 is the causative gene for hereditary recessive Parkinson's disease. Recent studies have reported that DJ-1 hydrolyzes cyclic 3-phosphoglyceric anhydride (cPGA), a highly reactive metabolite. However, the molecular mechanisms underlying cPGA hydrolase activity have yet to be fully elucidated. To gain a more comprehensive understanding of this activity in DJ-1, we performed molecular simulations that predicted how DJ-1 recognizes and hydrolyzes cPGA. The accuracy of these structural predictions was validated through systematic mutational analyses exemplified by loss of activity with the A107P mutation. Although DJ-1 possesses both cPGA hydrolase and α-oxoaldehyde hydratase activities in vitro, we confirmed that DJ-1 dysfunction caused an increase in cPGA-derived modifications but had no effect on α-oxoaldehyde-derived modifications in cells. Importantly, A107 and P158, pathogenic missense mutation sites found in Parkinson's disease patients, are critical for cPGA hydrolysis both in vitro and in cells. The evidence-based catalytic mechanism for DJ-1 hydrolysis of cPGA that we propose here explains their pathophysiological significance.
© 2025 Watanabe et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures








References
-
- Akhmadi, A., Yeskendir A., Dey N., Mussakhmetov A., Shatkenova Z., Kulyyassov A., Andreeva A., and Utepbergenov D.. 2024. DJ-1 protects proteins from acylation by catalyzing the hydrolysis of highly reactive cyclic 3-phosphoglyceric anhydride. Nat. Commun. 15:2004. 10.1038/s41467-024-46391-9 - DOI - PMC - PubMed
-
- Andreeva, A., Bekkhozhin Z., Omertassova N., Baizhumanov T., Yeltay G., Akhmetali M., Toibazar D., and Utepbergenov D.. 2019. The apparent deglycase activity of DJ-1 results from the conversion of free methylglyoxal present in fast equilibrium with hemithioacetals and hemiaminals. J. Biol. Chem. 294:18863–18872. 10.1074/jbc.RA119.011237 - DOI - PMC - PubMed
-
- Berendsen, H.J.C., Postma J.P.M., Vangunsteren W.F., Dinola A., and Haak J.R.. 1984. Molecular-dynamics with coupling to an external bath. J. Chem. Phys. 81:3684–3690. 10.1063/1.448118 - DOI
MeSH terms
Substances
Grants and funding
- JPMXP1323015483/Ministry of Education, Culture, Sports, Science and Technology
- JP22H02577/Japan Society for the Promotion of Science
- JP23H04923/Japan Society for the Promotion of Science
- JP18K14708/Japan Society for the Promotion of Science
- JP21K06161/Japan Society for the Promotion of Science
- JP24H01290/Japan Society for the Promotion of Science
- JP22H02562/Japan Society for the Promotion of Science
- JP24H02094/Japan Society for the Promotion of Science
- JP24ama121012/Japan Society for the Promotion of Science
- JP24ama121027/Japan Society for the Promotion of Science
- P18H02443/Japan Society for the Promotion of Science
- JP19H05712/Japan Society for the Promotion of Science
- JP22gm1410004/Japan Society for the Promotion of Science
- Basis for Supporting Innovative Drug Discovery and Life Science Research
- Takeda Science Foundation
- TMDU Nanken-Kyoten Foundation
- Tokushima University
- Institute of Science Tokyo
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

