Neonatal BCG vaccination to prevent asthma: Results from the MIS BAIR randomized controlled trial
- PMID: 40464744
- PMCID: PMC12136015
- DOI: 10.1111/pai.70110
Neonatal BCG vaccination to prevent asthma: Results from the MIS BAIR randomized controlled trial
Abstract
Background: Asthma has a significant impact worldwide, but prevention strategies remain limited. We aimed to evaluate the efficacy of neonatal BCG vaccination in preventing asthma by modulating early-life immunity.
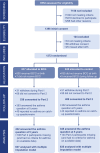
Methods: The Melbourne Infant Study: BCG for Allergy and Infection Reduction (MIS BAIR) was a phase 3 multicentre randomized controlled trial in Victoria, Australia. Infants were randomly assigned to receive the BCG-Denmark vaccine or no intervention within 10 days of birth. The incidence of asthma at 5 years of age was estimated using the International Study of Asthma and Allergies in Childhood questions.
Clinicaltrial: gov (NCT01906853).
Results: A total of 1272 infants were randomized. The adjusted incidence of asthma was 14.4% in the BCG group compared to 16.0% in the control group (adjusted risk difference [aRD] -1.7 percentage points; 95%CI -7.4, 3.9). Secondary outcomes, including severe asthma and use of preventer medication, showed similar trends, with an aRD of -3.9 (95%CI -7.7, 0.0), and -5.6 (95%CI -10.9, -0.4), respectively, favoring the BCG group. Among participants with one or both parents asthmatic, the rate of asthma was also lower in the BCG group (17.6%) compared with the control group (24.7%; aRD -7.2; 95%CI -15.9, 1.5), although a test for interaction was not significant (p = .07).
Conclusions: While the point estimates suggested BCG vaccination might protect against asthma, the wide uncertainty around the estimates means further studies with larger sample sizes are needed to evaluate the long-term benefits of BCG vaccination beyond its primary indication.
Keywords: BCG vaccine (Mycobacterium bovis); asthma; prevention; vaccine non‐specific off‐target effects; wheeze.
© 2025 The Author(s). Pediatric Allergy and Immunology published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Porsbjerg C, Melén E, Lehtimäki L, Shaw D. Asthma. Lancet. 2023;401(10379):858‐873. - PubMed
-
- Pollard AJ, Finn A, Curtis N. Non‐specific effects of vaccines: plausible and potentially important, but implications uncertain. Arch Dis Child. 2017;102(11):1077‐1081. - PubMed
-
- Benn CS, Fisker AB, Rieckmann A, Sorup S, Aaby P. Vaccinology: time to change the paradigm? Lancet Infect Dis. 2020;20(10):e274‐e283. - PubMed
-
- Freyne B, Curtis N. Does neonatal BCG vaccination prevent allergic disease in later life? Arch Dis Child. 2014;99(2):182‐184. - PubMed
-
- Kowalewicz‐Kulbat M, Locht C. BCG for the prevention and treatment of allergic asthma. Vaccine. 2021;39(50):7341‐7352. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- Ambizione grant (PZ00P3-209050)/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung
- Early Postdoc Mobility grant (P2GEP3_178155)/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung
- University of Melbourne
- Royal Children's Hospital Foundation
- GNT 1051228/National Health and Medical Research Council

