Perampanel as Add-on in Patients Aged ≥ 12 Years with Focal Epilepsy: A Prospective Real-World Observational Study from Southern China
- PMID: 40465107
- PMCID: PMC12255625
- DOI: 10.1007/s40120-025-00760-8
Perampanel as Add-on in Patients Aged ≥ 12 Years with Focal Epilepsy: A Prospective Real-World Observational Study from Southern China
Abstract
Introduction: This study aimed to evaluate the effectiveness, tolerability and safety of perampanel (PER) as an add-on therapy for southern Chinese patients aged ≥ 12 years with focal epilepsy.
Methods: This prospective cohort study enrolled consecutive patients with focal epilepsy treated between January 2023 and January 2024. Patients received PER as add-on therapy, with medication adjustments, seizure frequency and adverse events (AEs) monitored at 3, 6, 9 and 12 months. Logistic regression analyzed factors influencing 6- and 12-month treatment outcomes.
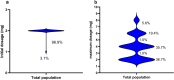
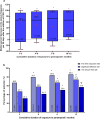
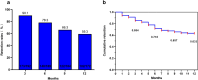
Results: Among 196 patients (full analysis set), 169 (86.2%) had drug-resistant epilepsy. Of these, 73.5% (144/196) received PER ≤ 4 mg/day. The 50% response rates at 6 and 12 months were 79.7% (114/143) and 86.0% (86/100), respectively. Retention rates at 6 and 12 months were 78.3% (148/189) and 59.3% (102/172), with cumulative retention rates of 71.8% and 63.3%, respectively. AEs occurred in 42 patients (21.4%), primarily dizziness and psychiatric symptoms. Logistic regression analysis identified disease duration of < 5 years (OR = 15.893, 95% CI = 1.418-178.158, P < 0.05) and unknown etiology (OR = 14.528, 95% CI = 2.508-84.140, P < 0.05) as predictors of higher long-term response rates.
Conclusion: PER demonstrated good effectiveness and safety as an add-on therapy for focal epilepsy in southern Chinese patients aged ≥ 12 years. Lower doses of PER (≤ 4 mg/day) may achieve satisfactory effectiveness in PER-sensitive populations, while shorter disease duration and unknown etiology were associated with better long-term outcomes. These findings support PER's utility in managing focal epilepsy, particularly in drug-resistant cases.
Keywords: Affect factors; Effectiveness; Focal epilepsy; Perampanel; Safety.
Plain language summary
This study evaluated the effectiveness, safety, and tolerability of perampanel (PER) as an adjunctive therapy for southern Chinese patients aged ≥ 12 years with focal epilepsy. A total of 196 patients, including 86.2% with drug-resistant epilepsy, were prospectively followed for 12 months. Most patients (73.5%) received a low PER dose (≤ 4 mg/day), which demonstrated comparable effectiveness to higher doses. At 6 and 12 months, 79.7% and 86.0% of patients, respectively, achieved a ≥ 50% reduction in seizure frequency. Retention rates were 78.3% at 6 months and 59.3% at 12 months. Adverse events, primarily dizziness and psychiatric symptoms, occurred in 21.4% of patients but were generally manageable. Logistic regression analysis identified shorter disease duration (< 5 years) and unknown etiology as significant predictors of better long-term outcomes. These findings support PER as an effective and well-tolerated adjunctive therapy for focal epilepsy, particularly in drug-resistant cases, with lower doses showing similar efficacy to higher doses.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Xiaoli Shi, Xiangru Lu, Lixia Li, Yanting Lu, Lang Shen, Jinou Zheng, Yuan Wu and Lu Yu declare that they have no conflict of interest. Ethical Approval: The study received approval from the Medical Ethics Committee of the First Affiliated Hospital of Guangxi Medical University and met the medical ethics requirements (2023-E350-01). Informed consent was obtained from all individual participants included in the study.
Figures
Similar articles
-
Levetiracetam add-on for drug-resistant focal epilepsy: an updated Cochrane Review.Cochrane Database Syst Rev. 2012 Sep 12;2012(9):CD001901. doi: 10.1002/14651858.CD001901.pub2. Cochrane Database Syst Rev. 2012. PMID: 22972056 Free PMC article.
-
Pregabalin add-on for drug-resistant focal epilepsy.Cochrane Database Syst Rev. 2022 Mar 29;3(3):CD005612. doi: 10.1002/14651858.CD005612.pub5. Cochrane Database Syst Rev. 2022. PMID: 35349176 Free PMC article.
-
Perampanel add-on for drug-resistant focal epilepsy.Cochrane Database Syst Rev. 2023 Apr 14;4(4):CD010961. doi: 10.1002/14651858.CD010961.pub2. Cochrane Database Syst Rev. 2023. PMID: 37059702 Free PMC article.
-
Effectiveness of perampanel as the only adjunctive anti-seizure medication in adults: Final results from the observational PERPRISE study in Germany.Epilepsia Open. 2025 Aug 21. doi: 10.1002/epi4.70117. Online ahead of print. Epilepsia Open. 2025. PMID: 40839459
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- Leo A, Giovannini G, Russo E, Meletti S. The role of AMPA receptors and their antagonists in status epilepticus. Epilepsia. 2018;59(6):1098–108. 10.1111/epi.14082:10.1111/epi.14082. - PubMed
-
- Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS. Progress report on new antiepileptic drugs: a summary of the Tenth Eilat Conference (EILAT X). Epilepsy Res. 2010;92(2–3):89–124. 10.1016/j.eplepsyres.2010.09.001. - PubMed
-
- Krauss GL, Bar M, Biton V, Klapper JA, Rektor I, Vaiciene-Magistris N, et al. Tolerability and safety of perampanel: two randomized dose-escalation studies. Acta Neurol Scand. 2012;125(1):8–15. 10.1111/j.1600-0404.2011.01588.x. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

