Insights on Hospitalisations from the Phase 3a ONWARDS 1-6 Trials of Once-Weekly Insulin Icodec
- PMID: 40465144
- PMCID: PMC12317947
- DOI: 10.1007/s13300-025-01745-4
Insights on Hospitalisations from the Phase 3a ONWARDS 1-6 Trials of Once-Weekly Insulin Icodec
Abstract
Introduction: The ONWARDS programme assessed the efficacy and safety of once-weekly insulin icodec (icodec) versus once-daily basal insulin comparators in type 2 diabetes (T2D) or type 1 diabetes (T1D). This post hoc exploratory analysis of ONWARDS 1-6 assessed the impact of icodec during and around hospitalisation.
Methods: ONWARDS 1-6 were randomised, two-arm, phase 3a trials (ClinicalTrials.gov: NCT04460885; NCT04770532; NCT04795531; NCT04880850; NCT04760626; NCT04848480). Adults with T2D (ONWARDS 1-5; n = 3765) and T1D (ONWARDS 6; n = 582) received icodec or once-daily comparators (insulin degludec, insulin glargine U100, insulin glargine U300). Hospitalised cases were analysed for: hospitalisation duration, icodec dose, self-measured blood glucose, glycated haemoglobin (HbA1c) levels, and clinically significant and severe hypoglycaemia before, during, and after hospitalisation.
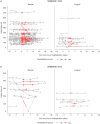
Results: Across trials, a similar number of participants receiving icodec (n = 152/2172) and once-daily comparators (n = 156/2175) were hospitalised. Median duration of hospital stay was similar between treatment groups (icodec, 5.0 days; once-daily comparators, 6.0 days); icodec dose remained fairly stable around hospitalisation. Most hospitalised participants completed the trial without permanently discontinuing treatment (icodec, 84.9%; once-daily comparators, 90.4%). Mean HbA1c levels remained relatively stable over assessed time points for both treatment groups. Six participants receiving icodec (one with T2D; five with T1D) and three receiving once-daily comparators (one with T2D; two with T1D) reported clinically significant or severe hypoglycaemia during hospitalisation.
Conclusions: Similar numbers of hospitalisations were reported in both treatment arms. Icodec treatment was continued during hospitalisation in most participants and did not appear to have an impact on glycaemic management or hypoglycaemia. This analysis suggests that once-weekly icodec could be managed in a similar way to once-daily basal insulin analogues during hospitalisation.
Trial registration: ClinicalTrials.gov identifiers, NCT04460885 (ONWARDS 1), NCT04770532 (ONWARDS 2), NCT04795531 (ONWARDS 3), NCT04880850 (ONWARDS 4), NCT04760626 (ONWARDS 5), NCT04848480 (ONWARDS 6).
Keywords: Hospitalisation; Hypoglycaemia; Insulin icodec; Type 1 diabetes; Type 2 diabetes.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Athena Philis-Tsimikas performs research and serves as an adviser on behalf of their employer for Abbott, Dexcom, Eli Lilly, Medtronic, Novo Nordisk, and Sanofi; there has been no direct or indirect transfer of funds. Julie Krogsdahl Bache, Ariel Fu, and Karen Salvesen-Sykes are employees of Novo Nordisk and hold stock in Novo Nordisk. Monika Kellerer received honoraria for speaker’s bureau or advisory board attendance with Abbott Diabetes Care, AstraZeneca, Bayer AG, Boehringer Ingelheim, Eli Lilly, MSD, Novo Nordisk, and Sanofi, and has also participated in randomised controlled trials funded by AstraZeneca, Eli Lilly, and Novo Nordisk. Stephen C. Bain received honoraria, teaching, and research sponsorship/grants from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, GSK, MSD, Novo Nordisk, Pfizer, Sanofi, and Takeda. Stephen C. Bain is an Editorial Board member of Diabetes Therapy. Stephen C. Bain was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. Ethical Approval: The ONWARDS 1–6 trials were conducted in compliance with the principles of the Declaration of Helsinki and in accordance with Good Clinical Practice guidelines of the International Conference on Harmonization [18, 19]. All relevant documents were reviewed and approved by the appropriate institutional review boards or independent ethics committees. All participants provided informed consent for participation in the trials and publication of data before enrolment. Further ethical approvals were not required for the present post hoc analysis, or de-identified case vignettes.
Figures

References
-
- Khalid JM, Raluy-Callado M, Curtis BH, Boye KS, Maguire A, Reaney M. Rates and risk of hospitalisation among patients with type 2 diabetes: retrospective cohort study using the UK General Practice Research Database linked to English Hospital Episode Statistics. Int J Clin Pract. 2014;68:40–8. - PMC - PubMed
-
- Comino EJ, Islam MF, Tran DT, et al. Association of processes of primary care and hospitalisation for people with diabetes: a record linkage study. Diabetes Res Clin Pract. 2015;108:296–305. - PubMed
-
- Rodriguez-Sanchez B, Cantarero-Prieto D. Socioeconomic differences in the associations between diabetes and hospital admission and mortality among older adults in Europe. Econ Hum Biol. 2019;33:89–100. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

