Simvastatin as Add-On Treatment to Escitalopram in Patients With Major Depression and Obesity: A Randomized Clinical Trial
- PMID: 40465256
- PMCID: PMC12138799
- DOI: 10.1001/jamapsychiatry.2025.0801
Simvastatin as Add-On Treatment to Escitalopram in Patients With Major Depression and Obesity: A Randomized Clinical Trial
Abstract
Importance: Major depressive disorder (MDD) and obesity are common noncommunicable disorders associated with substantial disease burden, which frequently occur comorbidly. Intriguingly, converging lines of evidence from animal models and genetic and observational studies have suggested a biological link between obesity, metabolic syndrome, and depression. Several small randomized clinical trials (RCTs) have suggested the antidepressive potential of statins.
Objective: To examine whether simvastatin added to escitalopram is efficacious in improving depressive symptoms compared with add-on placebo.
Design, setting, and participants: This was a confirmatory, double-blind, placebo-controlled, multicenter RCT. Adults with MDD and comorbid obesity from 9 tertiary care settings in Germany were enrolled in this analysis. Data were analyzed from July to October 2024.
Interventions: Simvastatin (40 mg per day) or placebo as add-on to escitalopram (10 mg for the first 2 weeks, then increased to 20 mg until the end of study) in a double-blind fashion for 12 weeks.
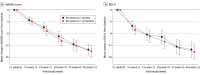
Main outcomes and measures: The primary outcome was change in Montgomery-Åsberg Depression Rating Scale (MADRS) score from baseline (week 0) to week 12.
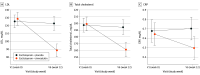
Results: From August 21, 2020, to June 06, 2024, a total of 161 patients were enrolled at 9 sites in Germany, of which 160 patients were included in the intention-to-treat analysis (placebo: n = 79, simvastatin: n = 81; mean [SD] age, 39.0 [11.0] years; 126 female [79%]). Retention in the trial was excellent (95.6%), and blinding was effectively maintained. There were 4 serious adverse events with no difference between the groups. Primary end point analysis in the intention-to-treat sample showed no significant treatment effect of add-on simvastatin in MADRS scores (mixed models for repeated measures least squares mean difference, 0.47 points; 95% CI, -2.08 to 3.02; P = .71). No effects of simvastatin treatment were observed in any of the mental health-related secondary end points. However, simvastatin treatment significantly reduced low-density lipoprotein cholesterol (simvastatin, -40.37 mg/dL; 95% CI, -47.41 to -33.33 mg/dL; placebo, -3.78 mg/dL; 95% CI, -11.18 to 3.62 mg/dL; P < .001), total cholesterol (simvastatin, -39.07 mg/dL; 95% CI, -49.42 to -28.73 mg/dL; placebo, -4.89 mg/dL; 95% CI, -15.64 to 5.87 mg/dL; P < .001), and C-reactive protein (simvastatin, -1.04 mg/L; 95% CI, -1.89 to -0.20 mg/L; placebo, 0.57 mg/L; 95% CI, -0.28 to 1.42 mg/L; P = .003) compared with placebo.
Conclusions and relevance: The study failed to meet its primary end point. This demonstrates that simvastatin did not exert additional antidepressive effects when added to escitalopram in patients with comorbid MDD and obesity, despite improving the cardiovascular risk profile.
Trial registration: ClinicalTrials.gov Identifier: NCT04301271.
Conflict of interest statement
Figures
References
-
- GBD 2019 Mental Disorders Collaborators . Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. 2022;9(2):137-150. doi: 10.1016/S2215-0366(21)00395-3 - DOI - PMC - PubMed
-
- GBD 2021 Diseases and Injuries Collaborators . Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2024;403(10440):2133-2161. doi: 10.1016/S0140-6736(24)00757-8 - DOI - PMC - PubMed
-
- World Health Organization . Obesity and overweight. Accessed June 10, 2024. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

