Cellular Adhesion Molecules and Adverse Outcomes in Chronic Heart Failure: Findings From the DAPA-HF Randomized Clinical Trial
- PMID: 40465275
- PMCID: PMC12138801
- DOI: 10.1001/jamacardio.2025.1592
Cellular Adhesion Molecules and Adverse Outcomes in Chronic Heart Failure: Findings From the DAPA-HF Randomized Clinical Trial
Abstract
Importance: Vascular cell adhesion molecule 1 (VCAM-1) and intracellular cell adhesion molecule 1 (ICAM-1) are responsible for immune cell-cell interactions. Systemic levels of VCAM-1 are associated with incident heart failure (HF).
Objectives: To determine if VCAM-1 and ICAM-1 levels are associated with progression of established HF.
Design, setting, and participants: Participants enrolled in the biomarker substudy of the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) randomized clinical trial had VCAM-1 and ICAM-1 levels measured at baseline and 12 months. The DAPA-HF trial was conducted at 410 sites in 20 countries. Patients with HF and reduced ejection fraction (HFrEF) in New York Heart Association (NYHA) class II to IV with elevated natriuretic peptides were enrolled between February 15, 2017, and August 17, 2018, with final follow-up on June 6, 2019. Data were analyzed from January 2023 to January 2025.
Interventions: Dapagliflozin, 10 mg, once daily vs placebo.
Main outcomes and measures: The primary outcome was the composite of a worsening HF event or cardiovascular death. The associations between VCAM-1 and ICAM-1 levels at baseline and the primary outcome, its components, and all-cause death were analyzed using Cox proportional hazards regression models adjusted for known prognostic variables including estimated glomerular filtration rate (eGFR), N-terminal pro-B-type natriuretic peptide (NT-proBNP), and high-sensitivity troponin T (hs-TnT), as well as high-sensitivity C-reactive protein.
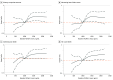
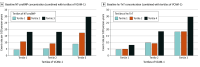
Results: A total of 3051 participants (mean [SD] age, 67.2 [10.5] years; 2386 male [78.2%]) were included in this study. Mean (SD) follow-up time was 17.6 (5.2) months. The median (IQR) baseline VCAM-1 level was 997 (816.7-1218.8) ng/mL. Compared with patients with lower concentrations of VCAM-1, those with higher concentrations of VCAM-1 were older (mean [SD] age T3 vs T1, 69.7 [9.7] years vs 64.1 [10.7] years; P < .001), in worse NYHA class (T3 vs T1, NYHA class III/IV 35.6% [362 of 1017] vs 26.5% [269 of 1017]; P < .001), and had higher NT-proBNP (median [IQR] T3 vs T1, 2018 [1126-3753] pg/mL vs 1118 [693-1830] pg/mL) and hs-TnT (median [IQR] T3 vs T1, 24.7 [17.1-37.5] ng/L vs 16.6 [11.6-24.9] ng/L) concentrations, and lower eGFR (mean [SD] T3 vs T1, 58.4 [17.6] mL/min/1.73 m2 vs 71.7 [18.0] mL/min/1.73 m2). Patients in tertile 3 of VCAM-1, compared with tertile 1, had the highest risk of each outcome (eg, adjusted hazard ratio [HR] for primary outcome 1.40; 95% CI, 1.11-1.77; P = .004). ICAM-1 level was not associated with an elevated risk of any outcome. The benefit of dapagliflozin vs placebo in reducing the risk of the primary outcome was consistent across VCAM-1 tertiles: HR, 0.76 (95% CI, 0.54-1.06), 0.82 (95% CI, 0.59-1.12), and 0.77 (95% CI, 0.61-0.98) for tertiles 1, 2 and 3, respectively (P for interaction = .93). There was no significant change in VCAM-1 level with dapagliflozin at 52 weeks.
Conclusions and relevance: Results of this substudy of the DAPA-HF randomized clinical trial demonstrate that higher VCAM-1 levels, possibly reflecting a distinct inflammatory/immune pathophysiological pathway in HFrEF, were associated with worse outcomes, even after adjustment for conventional prognostic variables.
Trial registration: ClinicalTrials.gov Identifier: NCT03036124.
Conflict of interest statement
Figures
Comment on
-
Vascular Cell Adhesion Molecule 1 in Heart Failure-Stuck on You.JAMA Cardiol. 2025 Aug 1;10(8):808-809. doi: 10.1001/jamacardio.2025.1600. JAMA Cardiol. 2025. PMID: 40465309 No abstract available.
Similar articles
-
Interleukin-6 in Heart Failure With Reduced Ejection Fraction and the Effect of Dapagliflozin: An Exploratory Analysis of the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure Trial.JACC Heart Fail. 2025 Jul;13(7):102393. doi: 10.1016/j.jchf.2024.12.012. Epub 2025 Mar 12. JACC Heart Fail. 2025. PMID: 40088234 Clinical Trial.
-
EuroQol 5-Dimension Questionnaire in Heart Failure With Reduced, Mildly Reduced, and Preserved Ejection Fraction: A Patient-Level Analysis of DAPA-HF and DELIVER.JACC Heart Fail. 2025 Feb;13(2):277-292. doi: 10.1016/j.jchf.2024.10.020. JACC Heart Fail. 2025. PMID: 39909641 Clinical Trial.
-
Sodium-Glucose Cotransporter 2 Inhibitor With and Without an Aldosterone Antagonist for Heart Failure With Preserved Ejection Fraction: The SOGALDI-PEF Trial.J Am Coll Cardiol. 2025 Aug 5;86(5):320-333. doi: 10.1016/j.jacc.2025.05.033. J Am Coll Cardiol. 2025. PMID: 40738559 Clinical Trial.
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

