Ibuprofen-Functionalized Alkyl α-hydroxy Methacrylate-Based Polymers
- PMID: 40465331
- PMCID: PMC12409833
- DOI: 10.1002/open.202500038
Ibuprofen-Functionalized Alkyl α-hydroxy Methacrylate-Based Polymers
Abstract
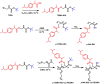
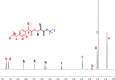
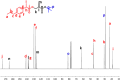
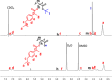
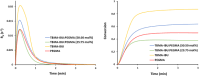
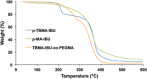
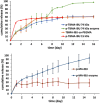
Novel alkyl α-hydroxymethacrylate-based prodrugs are prepared to improve bioavailability, decrease toxicity, and control drug release. First, an ibuprofen (IBU)-functionalized methacrylate (TBMA-IBU) is synthesized from the reaction of tert-butyl α-bromomethacrylate with IBU. The free radical homopolymerization and copolymerization with poly(ethylene glycol) methyl ether methacrylate (Mn = 300), cleavage of tert-butyl ester groups of the homopolymer and a copolymer with trifluoroacetic acid (TFA) gave new polymer prodrugs (p-TBMA-IBU, p-TBMA-IBU-co-PEGMA, p-MA-IBU, and p-MA-IBU-co-PEGMA), with IBU linked through ester bonds on the side chain. The release studies showed extended-release of IBU (20-60% in 15 d), which can be triggered under the stimulation of lipase. The in vitro studies indicate that the representative polymers are effective in relieving inflammatory responses in RAW264.7 macrophage cells without any cytotoxicity. These results suggest that the synthesized polymers with controllable functionality can be promising IBU prodrugs.
Keywords: alkyl α‐hydroxymethacrylates; drug design; ibuprofen; inflammation; polymer‐drug conjugates.
© 2025 The Author(s). ChemistryOpen published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
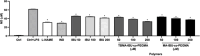
References
-
- Thakur S., Riyaz B., Patil A., Kaur A., Kapoor B., Mishra V., Biomed. Pharmacother. 2018, 106, 1011. - PubMed
-
- Peesa J. P., Yalavarthi P. R., Rasheed A., Mandava V. B. R., J. Acute Dis. 2016, 5, 364.
-
- Zawidlak‐Wegrzynska B., Kawalec M., Bosek I., Łuczyk Juzwa M., Adamus G., Rusin A., Filipczak P., Głowala‐Kosińska M., Wolańska K., Krawczyk Z., Kurcok P., Eur. J. Med. Chem. 2010, 45, 1833. - PubMed
-
- Yao M., Zhou W., Sangha S., Albert A., Chang A. J., Liu T. C., Wolfe M. M., Clin. Cancer Res. 2005, 11, 1618. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

