Trajectory-Ordered Objectives for Self-Supervised Representation Learning of Temporal Healthcare Data Using Transformers: Model Development and Evaluation Study
- PMID: 40465350
- PMCID: PMC12177421
- DOI: 10.2196/68138
Trajectory-Ordered Objectives for Self-Supervised Representation Learning of Temporal Healthcare Data Using Transformers: Model Development and Evaluation Study
Abstract
Background: The growing availability of electronic health records (EHRs) presents an opportunity to enhance patient care by uncovering hidden health risks and improving informed decisions through advanced deep learning methods. However, modeling EHR sequential data, that is, patient trajectories, is challenging due to the evolving relationships between diagnoses and treatments over time. Significant progress has been achieved using transformers and self-supervised learning. While BERT-inspired models using masked language modeling (MLM) capture EHR context, they often struggle with the complex temporal dynamics of disease progression and interventions.
Objective: This study aims to improve the modeling of EHR sequences by addressing the limitations of traditional transformer-based approaches in capturing complex temporal dependencies.
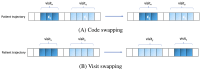
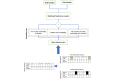
Methods: We introduce Trajectory Order Objective BERT (Bidirectional Encoder Representations from Transformers; TOO-BERT), a transformer-based model that advances the MLM pretraining approach by integrating a novel TOO to better learn the complex sequential dependencies between medical events. TOO-Bert enhanced the learned context by MLM by pretraining the model to distinguish ordered sequences of medical codes from permuted ones in a patient trajectory. The TOO is enhanced by a conditional selection process that focus on medical codes or visits that frequently occur together, to further improve contextual understanding and strengthen temporal awareness. We evaluate TOO-BERT on 2 extensive EHR datasets, MIMIC-IV hospitalization records and the Malmo Diet and Cancer Cohort (MDC)-comprising approximately 10 and 8 million medical codes, respectively. TOO-BERT is compared against conventional machine learning methods, a transformer trained from scratch, and a transformer pretrained on MLM in predicting heart failure (HF), Alzheimer disease (AD), and prolonged length of stay (PLS).
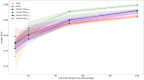
Results: TOO-BERT outperformed conventional machine learning methods and transformer-based approaches in HF, AD, and PLS prediction across both datasets. In the MDC dataset, TOO-BERT improved HF and AD prediction, increasing area under the receiver operating characteristic curve (AUC) scores from 67.7 and 69.5 with the MLM-pretrained Transformer to 73.9 and 71.9, respectively. In the MIMIC-IV dataset, TOO-BERT enhanced HF and PLS prediction, raising AUC scores from 86.2 and 60.2 with the MLM-pretrained Transformer to 89.8 and 60.4, respectively. Notably, TOO-BERT demonstrated strong performance in HF prediction even with limited fine-tuning data, achieving AUC scores of 0.877 and 0.823, compared to 0.839 and 0.799 for the MLM-pretrained Transformer, when fine-tuned on only 50% (442/884) and 20% (176/884) of the training data, respectively.
Conclusions: These findings demonstrate the effectiveness of integrating temporal ordering objectives into MLM-pretrained models, enabling deeper insights into the complex temporal relationships inherent in EHR data. Attention analysis further highlights TOO-BERT's capability to capture and represent sophisticated structural patterns within patient trajectories, offering a more nuanced understanding of disease progression.
Keywords: BERT; alzheimer disease; deep learning; disease prediction; effectiveness; electronic health record; heart failure; language mode; masked language mode; patient trajectories; prolonged health of stay; representation learning; temporal; transformer.
©Ali Amirahmadi, Farzaneh Etminani, Jonas Björk, Olle Melander, Mattias Ohlsson. Originally published in JMIR Medical Informatics (https://medinform.jmir.org), 04.06.2025.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures



References
-
- Miotto R, Li L, Kidd BA, Dudley JT. Deep patient: An unsupervised representation to predict the future of patients from the electronic health records. Sci Rep. 2016;6:26094. doi: 10.1038/srep26094. https://doi.org/10.1038/srep26094 srep26094 - DOI - DOI - PMC - PubMed
-
- Choi E, Bahadori M, Sun J, Kulas J, Schuetz A, Stewart W, Sun J. RETAIN: an interpretable predictive model for healthcare using reverse time attention mechanism. NIPS'16: Proceedings of the 30th International Conference on Neural Information Processing Systems; 2016 December 05; Red Hook, NY, United States. 2016. pp. 3512–3520.
-
- Ma F, Chitta R, Zhou J, You Q, Sun T, Gao J. Dipole: Diagnosis prediction in healthcare via attention-based bidirectional recurrent neural networks. KDD '17: Proceedings of the 23rd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining; 2017 August 13; NS, Halifax, Canada. 2017. pp. 1903–1911.
-
- Luo J, Ye M, Xiao C, Ma F. HiTANet: Hierarchical time-aware attention networks for risk prediction on electronic health records. KDD '20: Proceedings of the 26th ACM SIGKDD International Conference on Knowledge Discovery & Data Mining; 2020 August 20; CA, Virtual Event, USA. 2020. pp. 647–56.
-
- Xiao C, Choi E, Sun J. Opportunities and challenges in developing deep learning models using electronic health records data: a systematic review. J Am Med Inform Assoc. 2018;25(10):1419–1428. doi: 10.1093/jamia/ocy068. https://europepmc.org/abstract/MED/29893864 5035024 - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

