Examining the association between synaptic density and neurofibrillary tau among cognitively impaired and unimpaired older adults with and without Alzheimer's disease pathology
- PMID: 40465636
- PMCID: PMC12136093
- DOI: 10.1002/alz.70321
Examining the association between synaptic density and neurofibrillary tau among cognitively impaired and unimpaired older adults with and without Alzheimer's disease pathology
Abstract
Introduction: Synapse loss is a key driver of cognitive decline in Alzheimer's disease (AD), yet its direct relationship with neurofibrillary tau tangle (NFT) burden remains unclear. This study leveraged positron emission tomography (PET) imaging to investigate the link between NFT accumulation and synaptic density in older adults with and without AD pathology.
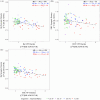
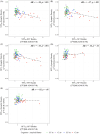
Methods: Older adults (N = 94) underwent PET imaging to quantify synaptic density ([11C]UCB-J distribution volume ratio [DVR]), Aβ plaque burden ([11C]PiB DVR), and NFT burden ([18F]MK-6240 standardized uptake value ratio). Analyses focused on NFT-synaptic density correlations in the hippocampus (Hp) and entorhinal cortex (ERC), with additional subgroup analyses based on cognitive and Aβ status.
Results: Hp NFT burden strongly correlated with synaptic density, while the ERC showed weaker effects. Subgroup analyses found robust Hp associations in unimpaired AD participants.
Conclusion: Hippocampal synaptic density is highly vulnerable to early NFT accumulation. SV2A PET imaging enables early detection of synaptic loss and may also identify resilience to AD pathology.
Highlights: Hippocampal synaptic density is similar in controls and unimpaired biologic AD. Hp synaptic density particularly vulnerable to Hp, ERC NFT burden. NFT-synaptic density relationship may vary between unimpaired and impaired biologic AD.
Keywords: Alzheimer's disease; SV2A; [11C]UCB‐J PET; neurofibrillary tau tangle; synaptic density.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Bradley T. Christian, Sterling C. Johnson, and Barbara B. Bendlin report grants from the National Institutes of Health for the conduct of the study. Sterling C. Johnson receives research funding from Cerveau Technologies. Barbara Bendlin received precursor and compounds from Avid Radiopharmaceuticals. Bradley T. Christian receives precursor and compounds from Avid and equipment from Cerveau Technologies and Lantheus. No other disclosures are reported. Author disclosures are available in the Supporting Information.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

