Predicting therapeutic clinical trial enrollment for adult patients with low- and high-grade glioma using supervised machine learning
- PMID: 40465719
- PMCID: PMC12136030
- DOI: 10.1126/sciadv.adt5708
Predicting therapeutic clinical trial enrollment for adult patients with low- and high-grade glioma using supervised machine learning
Abstract
Therapeutic clinical trial enrollment does not match glioma incidence across demographics. Traditional statistical methods have identified independent predictors of trial enrollment; however, our understanding of the interactions between these factors remains limited. To test the interactive effects of demographic, socioeconomic, and oncologic variables on trial enrollment, we designed boosted neural networks (BNNs) for all glioma patients (n = 1042), women (n = 445, 42.7%), and minorities (n = 151, 14.5%) and externally validated these models [whole cohort, n = 230; women, n = 89 (38.7%); minority, n = 66 (28.7%)]. For the whole-cohort BNN, the most influential variables on enrollment were oncologic variables, including KPS [total effect (TE), 0.327], chemotherapy (TE, 0.326), tumor location (TE, 0.322), and seizures (TE, 0.239). The women-only BNN exhibited a similar trend. Conversely, for the minority-only BNN, socioeconomic variables [insurance status (TE, 0.213), occupation classification (TE, 0.204), and employment status (TE, 0.150)] were most influential. These results may help prioritize patient-specific initiatives to increase accrual.
Figures

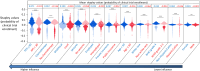
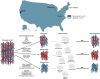
References
-
- Chang S. M., Barker F. G. II, Schmidt M. H., Sloan A. E., Kasper R., Phillips L., Shih K., Hariharan S., Berger M. S., The Glioma Outcomes Investigators , Clinical trial participation among patients enrolled in the Glioma Outcomes Project. Cancer 94, 2681–2687 (2002). - PubMed
-
- Reihl S. J., Patil N., Morshed R. A., Mehari M., Aabedi A., Chukwueke U. N., Porter A. B., Fontil V., Cioffi G., Waite K., Kruchko C., Ostrom Q., Barnholtz-Sloan J., Hervey-Jumper S. L., A population study of clinical trial accrual for women and minorities in neuro-oncology following the NIH Revitalization Act. Neuro Oncol. 24, 1341–1349 (2022). - PMC - PubMed
-
- Murthy V. H., Krumholz H. M., Gross C. P., Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA 291, 2720–2726 (2004). - PubMed
-
- US Congress, National Institutes of Health Revitalization Act of 1993, Public Law 103-43 (Washington, DC, 10 June 1993).
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

