Inhibition of acute lung inflammation by a neuroimmune circuit induced by vagal nerve stimulation
- PMID: 40465722
- PMCID: PMC12136045
- DOI: 10.1126/sciadv.adw7080
Inhibition of acute lung inflammation by a neuroimmune circuit induced by vagal nerve stimulation
Abstract
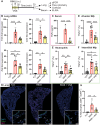
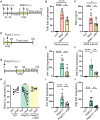
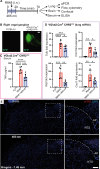
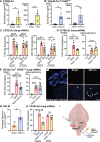
Vagus nerve stimulation (VNS) has been shown to limit immune cell activity across several pathologies ranging from sepsis to auto-immune diseases. While stimulation of vagal efferent neurons is known to reduce maladaptive host responses during endotoxemia, only selective vagal afferent neuron stimulation inhibited TLR7-induced macrophage activation and neutrophil recruitment to the lung. These anti-inflammatory actions are dependent on adrenal gland-derived epinephrine, as adrenalectomy or inhibition of epinephrine production eliminated the protection afforded by VNS. Selective afferent VNS induced activation in the nucleus tractus solitarius and the rostral ventrolateral medulla. Inhibition of neuronal activity in this brain region that controls peripheral sympathetic nervous system activity rendered VNS ineffective. Activation of the β2-adrenergic receptor (β2AR) is critical for innate immune cell suppression, as the anti-inflammatory effects of VNS were eliminated in β2AR knockout mice and with pharmacological inhibition of the β2AR. These findings demonstrate a previously unidentified neuroimmune circuit elicited by VNS that can control acute lung inflammation.
Figures
Update of
-
Inhibition of acute lung inflammation by a neuroimmune circuit induced by vagal nerve stimulation.bioRxiv [Preprint]. 2025 Feb 17:2025.02.12.637880. doi: 10.1101/2025.02.12.637880. bioRxiv. 2025. Update in: Sci Adv. 2025 Jun 6;11(23):eadw7080. doi: 10.1126/sciadv.adw7080. PMID: 40027796 Free PMC article. Updated. Preprint.
References
-
- Paules C., Subbarao K., Influenza. Lancet 390, 697–708 (2017). - PubMed
-
- Simoes E. A. F., Respiratory syncytial virus infection. Lancet 354, 847–852 (1999). - PubMed
-
- Peiris J. S. M., Yuen K. Y., Osterhaus A. D. M. E., Stöhr K., The severe acute respiratory syndrome. N. Engl. J. Med. 349, 2431–2441 (2003). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

