A cell-type-specific epigenetic mechanism encodes social investigatory behavior via Lrhcn1 and Hcn1
- PMID: 40465726
- PMCID: PMC12136044
- DOI: 10.1126/sciadv.adt5400
A cell-type-specific epigenetic mechanism encodes social investigatory behavior via Lrhcn1 and Hcn1
Abstract
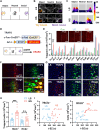
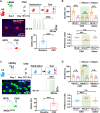
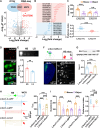
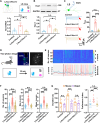
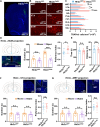
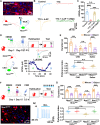
Social investigation is a complex behavior essential for understanding the surrounding world and is impaired in some major neuropsychiatric disorders. Yet, little is known about whether and, if yes, how social investigation is encoded in a specific type of cortical neurons. Here, we generated a computer vision tool to track social behavior of naïve mice and categorized them as high versus low social states. We integrated a genetically guided TRAP2 strategy with single-cell Ca2+ image and found that activity dynamics of serotonin receptor 2c (Htr2c)-expressing neurons in the prelimbic cortical layer 5 encode high social states. We uncovered a long noncoding RNA Lrhcn1 that controls Hcn1 transcription and regulates ensemble activity of Htr2c neurons, and this regulation motivates social investigation. Htr2c neurons directly and functionally project to nitric oxide synthase (Nos1) neurons in the hypothalamic nucleus. Gain- and loss-of function studies demonstrated that this projection specifically processes socially conveyed information.
Figures

References
-
- Baeyens F., Vansteenwegen D., De Houwer J., Crombez G., Observational conditioning of food valence in humans. Appetite 27, 235–250 (1996). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

