Low-dose radiation by radiopharmaceutical therapy enhances GD2 TRAC-CAR T cell efficacy in localized neuroblastoma
- PMID: 40465728
- PMCID: PMC12136040
- DOI: 10.1126/sciadv.adu4417
Low-dose radiation by radiopharmaceutical therapy enhances GD2 TRAC-CAR T cell efficacy in localized neuroblastoma
Abstract
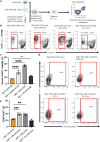
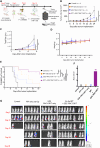
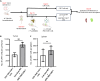
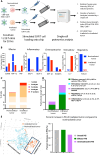
Chimeric antigen receptor (CAR) T cells have limited efficacy against solid tumors including neuroblastoma. Here, we evaluated whether low-dose radiation delivered by radiopharmaceutical therapy (RPT), known to potentiate immune checkpoint inhibitors, can synergize with CRISPR-edited GD2 TRAC-CAR T cells to improve outcomes in neuroblastoma. We found that in the localized model of neuroblastoma, low-dose radiation delivered by 177Lu-NM600, an alkylphosphocholine mimetic RPT agent, followed 9 days later by GD2 TRAC-CAR T cells led to complete tumor regression. Irradiation of neuroblastoma before GD2 TRAC-CAR T cells enhanced the release by CAR T cells of perforin, granzyme B, tumor necrosis factor-α, and interleukin-7 while abrogating transforming growth factor-β1. Low-dose RPT up-regulated the death receptor Fas on neuroblastoma, potentially enabling CAR-independent killing. This suggests that low-dose RPT can enhance suboptimal CAR T cell efficacy against solid tumors. However, optimization of radiation dose and timing may be needed for each patient and RPT agent to account for varied tumor radiosensitivity and dosimetry.
Figures



References
-
- Maude S. L., Laetsch T. W., Buechner J., Rives S., Boyer M., Bittencourt H., Bader P., Verneris M. R., Stefanski H. E., Myers G. D., Qayed M., De Moerloose B., Hiramatsu H., Schlis K., Davis K. L., Martin P. L., Nemecek E. R., Yanik G. A., Peters C., Baruchel A., Boissel N., Mechinaud F., Balduzzi A., Krueger J., June C. H., Levine B. L., Wood P., Taran T., Leung M., Mueller K. T., Zhang Y., Sen K., Lebwohl D., Pulsipher M. A., Grupp S. A., Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 378, 439–448 (2018). - PMC - PubMed
-
- Neelapu S. S., Locke F. L., Bartlett N. L., Lekakis L. J., Miklos D. B., Jacobson C. A., Braunschweig I., Oluwole O. O., Siddiqi T., Lin Y., Timmerman J. M., Stiff P. J., Friedberg J. W., Flinn I. W., Goy A., Hill B. T., Smith M. R., Deol A., Farooq U., McSweeney P., Munoz J., Avivi I., Castro J. E., Westin J. R., Chavez J. C., Ghobadi A., Komanduri K. V., Levy R., Jacobsen E. D., Witzig T. E., Reagan P., Bot A., Rossi J., Navale L., Jiang Y., Aycock J., Elias M., Chang D., Wiezorek J., Go W. Y., Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 377, 2531–2544 (2017). - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

