Senescence and inflammation are unintended adverse consequences of CRISPR-Cas9/AAV6-mediated gene editing in hematopoietic stem cells
- PMID: 40466639
- PMCID: PMC12208344
- DOI: 10.1016/j.xcrm.2025.102157
Senescence and inflammation are unintended adverse consequences of CRISPR-Cas9/AAV6-mediated gene editing in hematopoietic stem cells
Abstract
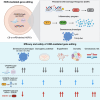
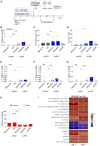
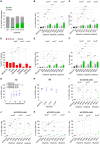
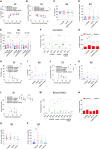
Gene editing (GE) using homology-directed repair (HDR) in hematopoietic stem and progenitor cells (HSPCs) offers promise for long-range gene correction of inherited genetic disorders. However, cellular responses induced by CRISPR-Cas9/AAV6 engineering impair the long-term repopulating potential of HDR-edited HSPCs, adversely impacting the safety and efficacy of clinical translation. Our study uncovers a durable senescence-like response in genetically engineered HSPCs triggered by p53 and interleukin (IL)-1/nuclear factor κB (NF-κB) activation, which restricts graft size and clonal diversity in long-term transplantation assays. We show that transient p53 inhibition or blocking inflammatory pathways mitigates senescence-associated responses, improving the repopulating capacity of edited HSPCs. Importantly, we identify treatment with Anakinra, an IL-1 signaling antagonist, as a promising strategy to enhance polyclonal output in HDR-edited cells while minimizing genotoxicity risks associated with the editing procedure. Overall, our findings present strategies to overcome key hurdles in HDR-based HSPC gene therapies, providing a framework for enhancing their efficacy and safety in clinical applications.
Keywords: CRISPR-Cas9; DNA damage; gene editing; gene therapy; genome integrity; hematopoietic stem cells; inflammatory programs; p53; senescence; viral vectors.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests R.D. Micco, A.C., L.d.V., F.M., L.N., and S.F. are inventors of patents on applications of gene editing in HSPCs owned and managed by the San Raffaele Scientific Institute and the Telethon Foundation. L.N. is a founder and quota holder of Genespire. T.C. is an inventor of patents on the applications of CAST-Seq.
Figures




References
-
- Ferrari S., Valeri E., Conti A., Scala S., Aprile A., Di Micco R., Kajaste-Rudnitski A., Montini E., Ferrari G., Aiuti A., Naldini L. Genetic engineering meets hematopoietic stem cell biology for next-generation gene therapy. Cell Stem Cell. 2023;30:549–570. doi: 10.1016/J.STEM.2023.04.014. - DOI - PubMed
-
- Porteus M.H. A New Class of Medicines through DNA Editing. N. Engl. J. Med. 2019;380:947–959. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

