DEPDC5 regulates the strength of excitatory synaptic transmission by interacting with ubiquitin-specific protease 46
- PMID: 40467011
- PMCID: PMC12165940
- DOI: 10.1016/j.nbd.2025.106985
DEPDC5 regulates the strength of excitatory synaptic transmission by interacting with ubiquitin-specific protease 46
Abstract
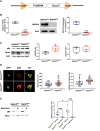
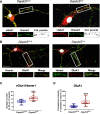
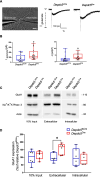
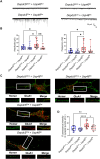
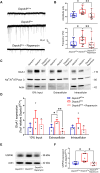
DEP-domain containing-5 (DEPDC5) is part of the GATOR1 complex that inhibits the mechanistic target of rapamycin complex-1 (mTORC1). Loss-of-function mutations in human DEPDC5 are the most common cause of lesional or non-lesional focal epilepsies associated with mTOR hyperactivation. Depdc5 silencing in mature neurons leads to excitation/inhibition imbalance and increased excitatory synapse strength. However, no link exists between mTORC1 hyperactivity and the increased activity of glutamatergic synapses. Here, we found that genetic deletion of Depdc5 in a conditional knockout (cKO) mouse recapitulates the excitatory/inhibitory imbalance observed after transient Depdc5 silencing, with increased strength of excitatory transmission and unaffected inhibitory transmission. In Depdc5 cKO neurons, the increased glutamate quantal size and response to exogenous glutamate are attributable to a higher density of GluA1-containing AMPA glutamate receptors due to a shift of the GluA1 subunit from the intracellular pool to the plasma membrane. The DEPDC5 protein interaction network included WDR48, WDR20, and USP46, a ubiquitin-specific protease that regulates GluA1, as key binding partners, along with previously established components of the mTORC1 signaling pathway. In the absence of DEPDC5, USP46 levels increase, and ubiquitination of GluA1 decreases accordingly. Either knockdown of USP46 or rapamycin treatment rescues both the increased glutamate quantal size and USP46 increase caused by Depdc5 deletion, indicating that USP46 overexpression depends on mTORC1 hyperactivity. The data indicate that the DEPDC5/mTORC1 system physiologically controls the excitatory strength by negatively modulating USP46 activity and AMPA receptor deubiquitination, and that failure of this effect can contribute to the development of the Depdc5-linked epileptic phenotype.
Keywords: AMPA receptors; DEPDC5 interactome; Depdc5 mutations; Deubiquitination; Excitatory synaptic strength; Primary neurons.
Copyright © 2024. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures



References
-
- Adelmant G., Calkins A.S., Garg B.K., Card J.D., Askenazi M., Miron A., Sobhian B., Zhang Y., Nakatani Y., Silver P.A., Iglehart J.D., Marto J.A., Lazaro J.B. DNA ends alter the molecular composition and localization of Ku multicomponent complexes. Mol. Cell. Proteomics. 2012;11:411–421. - PMC - PubMed
-
- Bacq A., Roussel D., Bonduelle T., Zagaglia S., Maletic M., Ribierre T., Adle-Biassette H., Marchal C., Jennesson M., An I., Genomics England Research Consortium, Picard F., Navarro V., Sisodiya S.M., Baulac S. Cardiac investigations in sudden unexpected death in DEPDC5-related epilepsy. Ann. Neurol. 2022;91:101–116. - PMC - PubMed
-
- Baldassari S., Picard F., Verbeek N.E., van Kempen M., Brilstra E.H., Lesca G., Conti V., Guerrini R., Bisulli F., Licchetta L., Pippucci T., Tinuper P., Hirsch E., de Saint Martin A., Chelly J., Rudolf G., Chipaux M., Ferrand-Sorbets S., Dorfmüller G., Sisodiya S., Balestrini S., Schoeler N., Hernandez-Hernandez L., Krithika S., Oegema R., Hagebeuk E., Gunning B., Deckers C., Berghuis B., Wegner I., Niks E., Jansen F.E., Braun K., de Jong D., Rubboli G., Talvik I., Sander V., Uldall P., Jacquemont M.L., Nava C., Leguern E., Julia S., Gambardella A., d’Orsi G., Crichiutti G., Faivre L., Darmency V., Benova B., Krsek P., Biraben A., Lebre A.S., Jennesson M., Sattar S., Marchal C., Nordli D.R., Jr., Lindstrom K., Striano P., Lomax L.B., Kiss C., Bartolomei F., Lepine A.F., Schoonjans A.S., Stouffs K., Jansen A., Panagiotakaki E., Ricard-Mousnier B., Thevenon J., de Bellescize J., Catenoix H., Dorn T., Zenker M., Müller-Schlüter K., Brandt C., Krey I., Polster T., Wolff M., Balci M., Rostasy K., Achaz G., Zacher P., Becher T., Cloppenborg T., Yuskaitis C.J., Weckhuysen S., Poduri A., Lemke J.R., Møller R.S., Baulac S. The landscape of epilepsy-related GATOR1 variants. Genet. Med. 2019;21:398–408. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

