Effect of dapagliflozin on metabolic dysfunction-associated steatohepatitis: multicentre, double blind, randomised, placebo controlled trial
- PMID: 40467095
- PMCID: PMC12135075
- DOI: 10.1136/bmj-2024-083735
Effect of dapagliflozin on metabolic dysfunction-associated steatohepatitis: multicentre, double blind, randomised, placebo controlled trial
Abstract
Objective: To assess the efficacy and safety of the sodium-glucose cotransporter 2 inhibitor dapagliflozin in participants with metabolic dysfunction-associated steatohepatitis (MASH).
Design: Multicentre, double blind, randomised, placebo controlled trial.
Setting: Six tertiary hospitals in China from 23 November 2018 to 28 March 2023.
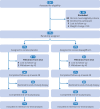
Participants: 154 adults with biopsy diagnosed MASH, with or without type 2 diabetes.
Interventions: All participants were randomly assigned to receive 10 mg orally of dapagliflozin or matching placebo once daily for 48 weeks.
Main outcome measures: The primary endpoint was MASH improvement (defined as a decrease of at least 2 points in non-alcoholic fatty liver disease activity score (NAS) or a NAS of ≤3 points) without worsening of liver fibrosis (defined as without increase of fibrosis stage) at 48 weeks. The secondary endpoints included the MASH resolution without worsening of fibrosis and fibrosis improvement without worsening of MASH. Analyses used the intention-to-treat dataset.
Results: MASH improvement without worsening of fibrosis was reported in 53% (41/78) of participants in the dapagliflozin group and 30% (23/76) in the placebo group (risk ratio 1.73 (95% confidence interval (CI) 1.16 to 2.58); P=0.006). Mean difference of NAS was -1.39 (95% CI -1.99 to -0.79); P<0.001). MASH resolution without worsening of fibrosis occurred in 23% (18/78) of participants in the dapagliflozin group and 8% (6/76) in the placebo group (risk ratio 2.91 (95% CI 1.22 to 6.97); P=0.01). Fibrosis improvement without worsening of MASH was reported in 45% (35/78) of participants in the dapagliflozin group, as compared with 20% (15/76) in the placebo group (risk ratio 2.25 (95% CI 1.35 to 3.75); P=0.001). The percentage of individuals who discontinued treatment because of adverse events was 1% (1/78) in the dapagliflozin group and 3% (2/76) in the placebo group.
Conclusion: Treatment with dapagliflozin resulted in a higher proportion of participants with MASH improvement without worsening of fibrosis, as well as MASH resolution without worsening of fibrosis and fibrosis improvement without worsening of MASH, than with placebo.
Trial registration: ClinicalTrials.gov NCT03723252.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: funding from Noncommunicable Chronic Diseases-National Science and Technology Major Project, National Science Fund for Distinguished Young Scholars, Joint Funds of the National Natural Science Foundation of China, Key-Area Clinical Research Program of Southern Medical University, and the National Key Research and Development Project; no support from any organizations for the submitted work (except those listed in the Funding section); no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical