Identification and characterisation of vaginal bacteria-glycan interactions implicated in reproductive tract health and pregnancy outcomes
- PMID: 40467588
- PMCID: PMC12137855
- DOI: 10.1038/s41467-025-60404-1
Identification and characterisation of vaginal bacteria-glycan interactions implicated in reproductive tract health and pregnancy outcomes
Abstract
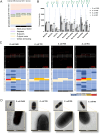
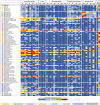
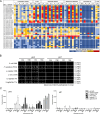
Lactobacillus displacement from the vaginal microbiome associates with adverse health outcomes and is linked to increased risk of preterm birth. Glycans mediate bacterial adhesion events involved in colonisation and infection. Using customised glycan microarrays, we establish glycan interaction profiles of vaginal bacteria implicated in reproductive health. Glycan binding signatures of the opportunistic pathogens Escherichia coli, Fusobacterium nucleatum and Streptococcus agalactiae to oligomannose N-glycans, galactose-terminating glycans and hyaluronic acid, respectively are highly distinct from Lactobacillus commensals. Binding to sulphated glycosaminoglycans by vaginal bacteria is pH dependent, as is binding to neutral and sialic acid-terminating glycans by F. nucleatum. Adhesion of Lactobacillus crispatus, Lactobacillus iners, Gardnerella vaginalis, S. agalactiae and F. nucleatum to vaginal epithelial cells is partially mediated by chondroitin sulphate. S. agalactiae binding to chondroitin sulphate C oligosaccharides is inhibited by L. crispatus. This study highlights glycans as mediators of vaginal bacterial binding events involved in reproductive health and disease.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
-
- van de Wijgert, J. & Jespers, V. The global health impact of vaginal dysbiosis. Res Microbiol168, 859–864 (2017). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

