A multisensor high-temperature signaling framework for triggering daytime thermomorphogenesis in Arabidopsis
- PMID: 40467614
- PMCID: PMC12137955
- DOI: 10.1038/s41467-025-60498-7
A multisensor high-temperature signaling framework for triggering daytime thermomorphogenesis in Arabidopsis
Abstract
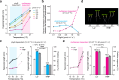
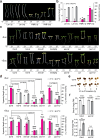
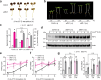
The phytochrome B (phyB) photoreceptor and EARLY FLOWERING 3 (ELF3) are two major plant thermosensors that monitor high temperatures primarily at night. However, high temperatures naturally occur during the daytime; the mechanism of daytime thermosensing and whether these thermosensors can also operate under intense sunlight remain ambiguous. Here, we show that phyB plays a substantial role in daytime thermosensing in Arabidopsis, and its thermosensing function becomes negligible only when the red light intensity reaches 50 μmol m-2 s-1. Leveraging this restrictive condition for phyB thermosensing, we reveal that triggering daytime thermomorphogenesis requires two additional thermosensory pathways. High temperatures induce starch breakdown in chloroplasts and the production of sucrose, which stabilizes the central thermal regulator PHYTOCHROME-INTERACTING FACTOR 4 (PIF4) by antagonizing phyB-dependent PIF4 degradation. In parallel, high temperatures release the inhibition of PIF4 transcription and PIF4 activity by ELF3. Thus, our study elucidates a multisensor high-temperature signaling framework for understanding diverse thermo-inducible plant behaviors in daylight.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
-
- Casal, J. J., Murcia, G. & Bianchimano, L. Plant thermosensors. Annu. Rev. Genet.58, 135–158 (2024). - PubMed
MeSH terms
Substances
Grants and funding
- R01GM087388/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
- CA-R-BPS-5186-H/United States Department of Agriculture | Agricultural Research Service (USDA Agricultural Research Service)
- R01GM132765/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
- MCB-2141560/NSF | BIO | Division of Molecular and Cellular Biosciences (MCB)
- R35 GM139598/GM/NIGMS NIH HHS/United States

