Effect of dolutegravir-based antiretroviral therapy on glycemic control in female mice
- PMID: 40467622
- PMCID: PMC12137651
- DOI: 10.1038/s41598-025-02130-8
Effect of dolutegravir-based antiretroviral therapy on glycemic control in female mice
Abstract
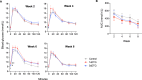
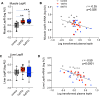
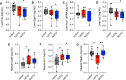
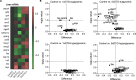
Dolutegravir (DTG), is recommended for all people with HIV, including pregnant women. Weight gain and hyperglycemia have been reported with DTG use, as well as a signal for neural tube defects (NTDs) that has waned over time. Obesity and hyperglycemia are risk factors for NTDs. We explored the impact of DTG-based antiretroviral therapy (ART) on weight gain and glucose homeostasis in female mice. C57BL/6 mice were treated daily for 9 weeks with water (control), 1xDTG (2.5 mg/kg DTG + 33.3/50 mg/kg emtricitabine/tenofovir-yielding therapeutic levels), or 5xDTG (12.5 mg/kg + 33.3/50 mg/kg emtricitabine/tenofovir). Overnight fasted glucose, weight, and oral glucose tolerance test (OGTT) were measured at 2-8 weeks. Tissue was collected for expression analyses of glucose homeostasis pathways. Weight gain was similar between groups. We observed a transient fasted hyperglycemia with DTG treatment, that peaked at week 6 and resolved by week 9. No significant differences were observed in insulin or OGTT response between groups. DTG was associated with a gradual and persistent decrease in plasma leptin and increase in plasma corticosterone levels compared to controls. Downregulation of genes involved in gluconeogenesis and lipogenesis in liver were observed in DTG-treated mice that remained euglycemic. Muscle and liver leptin receptor expression was elevated with DTG treatment. DTG was associated with transient hyperglycemia, lower leptin and higher corticosterone. Induction of compensatory mechanisms may have aided to restore/maintain euglycemia. This transient nature of the glycemic dysregulation may in part explain the loss of the NTD signal that was observed at the initial roll out of DTG but waned over time.
Keywords: Cortisol; Hyperglycemia; INSTI; Leptin; Lipogenesis; Neural tube defects.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
Long-term metabolic changes with bictegravir/emtricitabine/tenofovir alafenamide or dolutegravir-containing regimens for HIV.AIDS Res Ther. 2025 Apr 7;22(1):45. doi: 10.1186/s12981-025-00732-w. AIDS Res Ther. 2025. PMID: 40197415 Free PMC article. Clinical Trial.
-
Change in weight and BMI associated with switching to bictegravir/emtricitabine/tenofovir alafenamide versus a dolutegravir-based regimen among virologically suppressed adults living with HIV through 144 weeks.Medicine (Baltimore). 2025 Mar 7;104(10):e41728. doi: 10.1097/MD.0000000000041728. Medicine (Baltimore). 2025. PMID: 40068072 Free PMC article.
-
Safety and efficacy of lamivudine/dolutegravir vs. bictegravir/emtricitabine/tenofovir alafenamide in antiretroviral-naive adults with HIV-1 infection in Shanghai, China: a single-centre retrospective study.J Med Microbiol. 2025 Jan;74(1). doi: 10.1099/jmm.0.001949. J Med Microbiol. 2025. PMID: 39773780
-
Doing More With Less: Review of Dolutegravir-Lamivudine, a Novel Single-Tablet Regimen for Antiretroviral-Naïve Adults With HIV-1 Infection.Ann Pharmacother. 2020 Dec;54(12):1252-1259. doi: 10.1177/1060028020933772. Epub 2020 Jun 9. Ann Pharmacother. 2020. PMID: 32517480 Review.
-
Prevalence of Emergent Dolutegravir Resistance Mutations in People Living with HIV: A Rapid Scoping Review.Viruses. 2024 Mar 4;16(3):399. doi: 10.3390/v16030399. Viruses. 2024. PMID: 38543764 Free PMC article.
References
-
- Pandey, A. & Galvani, A. P. The global burden of HIV and prospects for control. Lancet HIV.6, e809–e811 (2019). - PubMed
-
- Gandhi, R. T. et al. Antiretroviral drugs for treatment and prevention of hiv infection in adults: 2022 recommendations of the international antiviral society-USA panel. JAMA329, 63–84 (2023). - PubMed
-
- Consolidated guidelines on HIV prevention. testing, treatment, service delivery and monitoring: recommendations for a public health approach (World Health Organization, 2021). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

