Chronic social isolation-unpredictable stress induces early-onset cognitive deficits and exacerbates Aβ accumulation in the 5xFAD mouse model of Alzheimer's disease
- PMID: 40467855
- PMCID: PMC12436184
- DOI: 10.1038/s41380-025-03067-0
Chronic social isolation-unpredictable stress induces early-onset cognitive deficits and exacerbates Aβ accumulation in the 5xFAD mouse model of Alzheimer's disease
Abstract
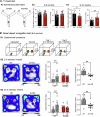
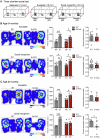
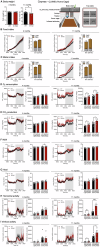
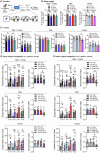
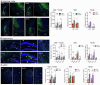
Aging and genetic predisposition are the primary risk factors for Alzheimer's disease (AD), while chronic stress represents a modifiable risk factor that can accelerate aging and drive AD progression. However, the complex interplay between aging, chronic stress and genetic underpinnings in AD pathogenesis remains poorly understood. Notably, cognitive phenotyping in AD mouse models has yielded inconsistent results. In this study, we characterized the age-dependent trajectory of phenotypes in 5xFAD mice on a congenic C57BL/6 J background. These mice harbor five familial AD (FAD)-related mutations in the amyloid precursor protein (APP) and presenilin 1 (PSEN1) genes. Aβ plaque deposition was detected in specific brain regions by 4 months of age, but cognitive performance remained intact at this stage. However, by 8-9 months, these mice developed impairments in spatial working memory, novel object recognition memory, and social recognition memory. By 11 months, they also showed metabolic alterations, including lower body weight, higher energy expenditure and increased locomotor activity. Furthermore, after 10 days of chronic social isolation-unpredictable stress, 4-month-old 5xFAD mice exhibited cognitive deficits, accompanied by increased Aβ accumulation in the medial prefrontal cortex, hippocampus, and entorhinal cortex. In contrast, age- and sex-matched wild-type littermate controls subjected to the same stress paradigm showed no significant cognitive changes. These observations suggest that Aβ deposition increases stress vulnerability in 5xFAD mice. We conclude that the phenotypic expression of AD-related gene mutations, including pathological changes and cognitive decline, progresses with age and can be induced by chronic psychological stress, underscoring the interactive effects of stress, aging, and genetic vulnerability on disease onset and severity.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval: All animal procedures were approved by the Institutional Animal Care and Use Committee of Augusta University (Animal Use Protocol #2017-0868) and conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Figures

References
-
- Chartier-Harlin MC, Crawford F, Houlden H, Warren A, Hughes D, Fidani L, et al. Early-onset Alzheimer’s disease caused by mutations at codon 717 of the beta-amyloid precursor protein gene. Nature. 1991;353:844–6. - PubMed
-
- Goate A, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, Fidani L, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature. 1991;349:704–6. - PubMed
-
- Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature. 1995;375:754–60. - PubMed
-
- Rogaev EI, Sherrington R, Rogaeva EA, Levesque G, Ikeda M, Liang Y, et al. Familial Alzheimer’s disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer’s disease type 3 gene. Nature. 1995;376:775–8. - PubMed
MeSH terms
Substances
Grants and funding
- AG076235/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- AG062166/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- AG083841/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- R01 AG083841/AG/NIA NIH HHS/United States
- R01 AG076235/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

