Chemoorganoautotrophic lifestyle of the anaerobic enrichment culture N47 growing on naphthalene
- PMID: 40467871
- PMCID: PMC12137691
- DOI: 10.1038/s42003-025-08172-y
Chemoorganoautotrophic lifestyle of the anaerobic enrichment culture N47 growing on naphthalene
Abstract
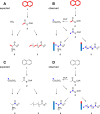
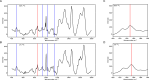
In almost all respiratory organisms, organic substrates are degraded via catabolic processes to the central metabolite acetyl-CoA which is then oxidized to CO2 for energy metabolism or used as a building block for anabolism. Most microorganisms have either the closed tricarboxylic acid cycle or the complete Wood-Ljungdahl-pathway for acetyl-CoA oxidation, but the sulfate-reducing, naphthalene-degrading culture N47 possesses both completely. Combining 13C- labeled substrates and mass-specific GC-MS analysis of amino acids and fatty acids with enzyme activity assays suggests that N47 has a chemoorganoautotrophic metabolism degrading complex organic substrates such as naphthalene. Surprisingly, however, the biomass is mainly produced from acetyl-CoA generated de novo via CO2-fixation. This metabolism probably requires both a complete Wood-Ljungdahl pathway for acetyl-CoA oxidation and a reverse tricarboxylic acid cycle for CO2 fixation. Based on genome analysis, this chemoorganoautotrophic metabolism seems to also occur in other sulfate-reducers and anaerobic ammonium-oxidizers.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
-
- Thauer, R., Zinkhan, D. & Spormann, A. Biochemistry of acetate catabolism in anaerobic chemotrophic bacteria. Ann. Rev. Microbiol.43, 43–67 (1989). - PubMed
-
- Thauer, R. K. Citric-acid cycle, 50 years on: modifications and an alternative pathway in anaerobic bacteria. Eur. J. Biochem.176, 497–508 (1988). - PubMed
-
- Meckenstock, R. U. et al. Anaerobic degradation of benzene and polycyclic aromatic hydrocarbons. J. Mol. Microbiol. Biotechnol.26, 92–118 (2016). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

