Effectiveness of polyethylene glycol and glutaraldehyde as enhancers for lipase-immobilized hybrid organic-inorganic nanoflowers
- PMID: 40467888
- PMCID: PMC12234603
- DOI: 10.1007/s00449-025-03181-x
Effectiveness of polyethylene glycol and glutaraldehyde as enhancers for lipase-immobilized hybrid organic-inorganic nanoflowers
Abstract
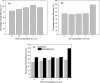
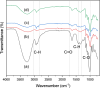
The present study investigates the influence of polyethylene glycol (PEG) and glutaraldehyde (GA) on the synthesis and enzymatic activity of lipase hybrid nanoflowers. The effect of lipase concentration on hybrid nanoflower formation was first assessed, revealing that the optimum lipase concentration was 0.2 mg/mL. At this concentration, the encapsulation of lipase within the hybrid nanoflowers reached its maximum efficiency. Further, the effects of PEG and GA concentrations, as well as pH, on the enzymatic activity of the nanoflowers were evaluated. The results demonstrated that 2% (v/v) PEG and 3% (v/v) GA were the most effective concentrations, with the highest activity observed at pH 8. Comparative studies showed that GA-treated lipase hybrid nanoflowers exhibited a remarkable 160% increase in enzymatic activity over the free lipase, outperforming PEG in terms of catalytic performance. Scanning Electron Microscopy (SEM) and Fourier Transform Infrared (FTIR) spectroscopy analyses confirmed that both PEG and GA treatments altered the morphology and structural characteristics of the hybrid nanoflowers, with GA inducing more pronounced changes. Despite these morphological alterations, the enzymatic activity was significantly enhanced, particularly in the GA-treated hybrid nanoflowers. In conclusion, this study highlights the superior performance of glutaraldehyde as an enhancer for the production of highly active lipase hybrid nanoflowers, offering promising applications in biocatalysis and enzyme immobilization.
Keywords: Glutaraldehyde; Lipase hybrid nanoflowers; Polyethylene glycol.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare no competing interests. Ethical approval: The present research study does not involve any human participants, their data, or biological. Consent to participate: Not applicable. Consent to publish: Not applicable.
Figures









References
-
- Chapman J, Ismail AE, Dinu CZ (2018) Catalysts 8:238. 10.3390/catal8060238
-
- Bell EL, Finnigan W, France SP, Green AP, Hayes MA, Hepworth LJ, Lovelock SL, Niikura H, Osuna S, Romero E, Ryan KS, Turner NJ, Flitsch SL (2021). Nat Rev Methods Prim. 10.1038/s43586-021-00044-z
-
- Filho DG, Silva AG, Guidini CZ (2019). Appl Microbiol Biotechnol. 10.1007/s00253-019-10027-6 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

