Atezolizumab plus paclitaxel and bevacizumab as first-line treatment of advanced triple-negative breast cancer: the ATRACTIB phase 2 trial
- PMID: 40467896
- PMCID: PMC12353800
- DOI: 10.1038/s41591-025-03734-3
Atezolizumab plus paclitaxel and bevacizumab as first-line treatment of advanced triple-negative breast cancer: the ATRACTIB phase 2 trial
Abstract
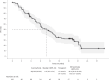
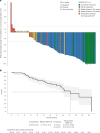
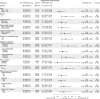
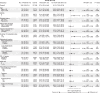
Triple-negative breast cancer (TNBC) is a highly aggressive subtype of breast cancer with poor prognosis. The current first-line treatment for advanced TNBC (aTNBC) is determined by the expression of programmed cell death-ligand 1 (PD-L1). In the ATRACTIB trial-a multicenter, single-arm, phase 2 study-we evaluated the combination of atezolizumab, paclitaxel and bevacizumab as first-line treatment for patients with aTNBC, independently of PD-L1 status. The primary endpoint was investigator-assessed progression-free survival. One hundred female patients were enrolled, with most evaluable tumors being PD-L1-negative (97.6%). The primary endpoint was met, with a median progression-free survival of 11.0 months (95% confidence interval (CI): 9.0-13.4; P < 0.001). The objective response rate was 63.0% (95% CI: 52.8-72.4) and median overall survival was 27.4 months (95% CI: 23.4-37.4). No treatment-related deaths or new safety signals were observed. This combination demonstrated significant antitumor activity as first-line therapy for aTNBC patients and merits further investigation. ClinicalTrials.gov Identifier: NCT04408118 .
© 2025. The Author(s).
Conflict of interest statement
Competing interests: M.G. reports having received honoraria from Novartis, Gilead, AstraZeneca and Pfizer; having personal support for attending meetings and/or travel from Roche, Pfizer, AstraZeneca and Gilead; and having received honoraria for advisory board participation from Gilead, Novartis, AstraZeneca and Pfizer. I.B. reports having received honoraria as Medical Monitor from MEDSIR; having received honoraria for advisory board participation from AstraZeneca, Bristol-Myers Squibb, Celgene, Daiichi Sankyo, Eisai, Gilead, Grünenthal, GSK, Jazz Pharmaceutical, Lilly, MSD, Novartis, Pfizer, Pierre-Fabre, Roche, Seagen and Veracyte; having personal support for attending meetings and/or travel from AstraZeneca, Bristol-Myers Squibb, Daiichi Sankyo, Gilead, Lilly, Novartis, Pfizer, Pierre-Fabre and Roche; and having received institutional financial support from Agendia, AstraZeneca, Lilly, Pfizer and Roche. A.C.-S. reports having received personal honoraria for advisory board participation from GlaxoSmithKline and AstraZeneca; having personal honoraria for speakers’ bureaus from GlaxoSmithKline, AstraZeneca, MSD, Eisai, Accord Healthcare, Pfizer, and Pharma&; having personal support for attending meetings and/or travel from Pfizer, GlaxoSmithKline and MSD. They are also founder of ONCARE Madrid. F.M. reports having consulting fees from AstraZeneca, Clovis, Daiichi Sankyo, EISAI, Gilead, GlaxoSmithKline, Novartis, Myriad Genetic, Seagen, Stemline Menarini, Lilly, MSD, Pfizer, Roche, Bionteck and Nerviano; having personal honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events for AstraZeneca, Clovis, Daiichi Sankyo, EISAI, Gilead, GlaxoSmithKline, Myriad Genetic, Seagen, Stemline Menarini, Lilly, MSD, Pfizer and Roche; having personal support for attending meetings and/or travel from AstraZeneca, Daiichi Sankyo, Gilead, GlaxoSmithKline, Stemline Menarini, Lilly, Pfizer and Roche; and having received honoraria for advisory board participation from Immutep, Amgen and Palleos. S.M. has received personal support for attending meetings and/or travel from AstraZeneca, Daiichi Sankyo, Gilead, Lilly, Novartis, MSD and Pfizer; and has received institutional financial support from Lilly. I.C.-P. reports having received personal honoraria as consultant, advisor and speaker from Gilead, MSD, Roche and AstraZeneca; having received personal honoraria for educational events from Roche and Daiichi Sankyo; and having received institutional financial support from Roche. S.R. reports having personal honoraria for speakers’ bureaus from Roche; and having personal support for attending meetings and/or travel from Accord Healthcare and Lilly. A.M.-B. reports having personal support for attending meetings and/or travel from GlaxoSmithKline, MSD and Roche; having received personal honoraria for speaker participation from AstraZeneca, GlaxoSmithKline and Seagen; and has received institutional financial support from GlaxoSmithKline. E.L. reports having received personal honoraria from Pfizer, Lilly, Novartis, Gilead, Daichii and AstraZeneca; and having received personal honoraria for advisory board participation from Gilead and Daichii. M.T.T. reports having received honoraria for local meetings from AstraZeneca, Bristol-Myers Squibb, Celgene, Daiichi Sankyo, Eisai, Gilead, Grünenthal, GlaxoSmithKline, Lilly, MSD, Novartis, Pfizer, Pierre-Fabre and Roche; and having personal support for attending meetings and/or travel from Merck, Pzifer and Servier. M. de L. reports having received honoraria for lectures, presentations, speakers bureaus, manuscript writing and educational events from Eli Lilly, Novartis, Seagen, Takeda, Roche, Daiichi Sankyo, Tomalab, Gilead, Genetic, Menarini, Sophos and Istituto Gentili; having received economical support for attending meetings and/or travel from Gilead, Novartis, Roche and AstraZeneca; and having participated on a Data Safety Monitoring Board or Advisory Board for Pfizer, AstraZeneca, Sanofi, Seagen, Novartis, Ipsen, Roche, Pierre-Fabre, Daiichi Sankyo, GSK, MSD and Menarini. S.G.-V., J.A.G., O.B., J.R.-M. and M.S.-C. are full-time employees at MEDSIR. G.A. reports having personal support for attending meetings and/or travel along with personal honoraria from MEDSIR. J.M.P.-G. reports having received personal honoraria for advisory board participation from Lilly, Roche, Eisai, Daichii Sankyo, AstraZeneca, Seattle Genetics, MSD and Gilead; having personal support for attending meetings and/or travel from Roche; and being an employee at MEDSIR. J.C. reports being a consultant/advisor for Roche, AstraZeneca, Seattle Genetics, Daiichi Sankyo, Lilly, Merck Sharp & Dohme, Leuko, Bioasis, Clovis Oncology, Boehringer Ingelheim, Ellipses, Hibercell, BioInvent, Gemoab, Gilead, Menarini, Zymeworks, Reveal Genomics, Scorpion Therapeutics, Expres2ion Biotechnologies, Jazz Pharmaceuticals, Abbvie, BridgeBio, Biontech, Biocon, Circle Pharma, Delcath Systems, Inc., Hexagon Bio; receiving honoraria from Roche, Novartis, Eisai, Pfizer, Lilly, Merck Sharp & Dohme, Astrazeneca, Gilead, Steamline Therapeutics and Daiichi Sankyo; has stock of MAJ3 Capital and Leuko (relative); received travel, accommodation and expenses from Roche, Novartis, Eisai, Pfizer, Daiichi Sankyo, Astrazeneca, Gilead Merck Sharp & Dhome, Steamline Therapeutics; and has the following patents: Pharmaceutical Combinations of A Pi3k Inhibitor and a Microtubule Destabilizing Agent. Javier Cortés Castán, Alejandro Piris Giménez, Violeta Serra Elizalde. WO 2014/199294A—Issued, Her2 as a predictor of response to dual HER2 blockade in the absence of cytotoxic therapy. Aleix Prat, Antonio Llombart, Javier Cortés.US 2019/ 0338368 A1—Licensed. J.C. also reports their institution received research funding from Roche, Ariad Pharmaceuticals, AstraZeneca, Baxalta GMBH/Servier Affaires, Bayer Healthcare, Eisai, F. Hoffman-La Roche, Guardanth Health, Merck Sharp & Dohme, Pfizer, Piqur Therapeutics, Puma C and Queen Mary University of London. A.L.-C. reports receiving research support from Roche, Agendia, Lilly, Pfizer, Novartis, Merck Sharp & Dohme, Gilead and Daichii-Sanyo; consulting or advisory role for Lilly, Roche, Pfizer and Novartis; speakers’ bureaus from Lilly, AstraZeneca, Pfizer, Novartis and Merck Sharp & Dohme; travel support from Roche, Pfizer, Steamline Therapeutics, Merck Sharp & Dhome and AstraZeneca; and stock or other ownership of MAJ3 Capital and Initia-Research. The other authors declare no competing interests.
Figures

References
-
- Gennari, A. et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann. Oncol.32, 1475–1495 (2021). - PubMed
-
- Surveillance Research Program, National Cancer Institute. SEER*Explorer: An Interactive Website for SEER Cancer Statistics (SEER Incidence Data, November 2024, accessed 16 May 2025); https://seer.cancer.gov/statistics-network/explorer
-
- Dent, R. et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin. Cancer Res.13, 4429–4434 (2007). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

