Distribution characteristics of microbial aerosols and optimization of protective methods during ultrasonic scaling procedure
- PMID: 40467899
- PMCID: PMC12137542
- DOI: 10.1038/s41598-025-04857-w
Distribution characteristics of microbial aerosols and optimization of protective methods during ultrasonic scaling procedure
Abstract
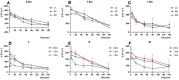
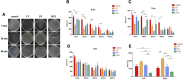
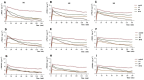
This study aimed to explore microbial aerosol distribution characteristics in the dental clinic during ultrasonic scaling and evaluate the effects of three different interventions on aerosol distribution and protective effects. For twenty minutes, ultrasonic scaling was carried out in a standardized operatory room. A blank control group and three intervention groups were created: high-volume evacuator (HVE), plasma purification (PP), and fenestrated ventilation (VT). The mass concentration of PM1.0, PM2.5, and PM10.0 aerosol particles was tracked in real time, and colony counts were calculated using air deposition. After ultrasonic scaling, there was a significant increase in aerosol dispersion of various particle sizes and distribution within a 1.5-m radius of the core area (P < 0.05). The number of colonies in each group varied over time at 0.5 and 1.0 m from the patient's head, but there was no significant difference at 1.5 m (P > 0.05). The PP group demonstrated the greatest decrease in aerosol mass concentration difference. The VT group initially had the lowest aerosol mass concentration difference, but with a slight decrease. The aerosol mass concentration difference between the HVE groups grew with distance. Traditional ultrasonic scaling poses a risk of aerosol contamination during and after treatment. The operatory room's air can be efficiently purified by plasma purification, which maintains lower levels of aerosol particle size than other groups. Microbial aerosols created by ultrasonic scaling can be quickly reduced by ventilation. At close range, the high-volume evacuator can lower the risk of infection while the benefit diminishes as the distance increases.Trial registration: This study was registered on the website of China Clinical Trial Registration Center (ChiCTR2400090751) (12/10/2024).
Keywords: Aerosol; High-volume evacuator; Plasma purification; Ultrasonic scaling.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Human ethics and consent to participate: This study was initiated after getting approval from the institutional ethics committee of Zhejiang Chinese Medical University and Hospital, Hangzhou, Zhejiang Consent for publication (No. ZCMUHSIRB-2024081312), and all methods were performed in accordance with the relevant guidelines and regulations. Consent to participate: Written informed consent was obtained from all the participants prior to the enrollment of this study.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

