Increased CSF drainage by non-invasive manipulation of cervical lymphatics
- PMID: 40468071
- PMCID: PMC12267054
- DOI: 10.1038/s41586-025-09052-5
Increased CSF drainage by non-invasive manipulation of cervical lymphatics
Abstract
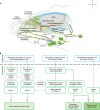
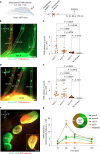
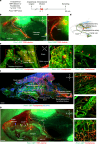
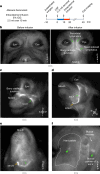
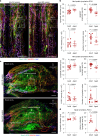
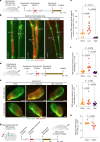
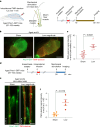
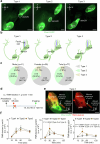
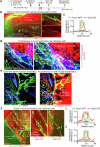
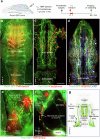
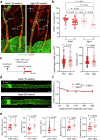
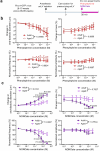
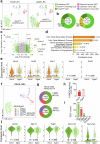
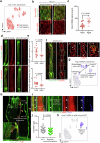
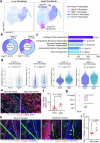
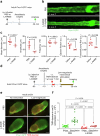
Cerebrospinal fluid (CSF) in the subarachnoid space around the brain drains to lymph nodes in the neck, but the connections and regulation have been challenging to identify1-24. Here we used fluorescent tracers in Prox1-GFP lymphatic reporter mice to map the pathway of CSF outflow through lymphatics to superficial cervical lymph nodes. CSF entered initial lymphatics in the meninges at the skull base and continued through extracranial periorbital, olfactory, nasopharyngeal and hard palate lymphatics, and then through smooth muscle-covered superficial cervical lymphatics to submandibular lymph nodes. Tracer studies in adult mice revealed that a substantial amount of total CSF outflow to the neck drained to superficial cervical lymph nodes. However, aged mice had fewer lymphatics in the nasal mucosa and hard palate and reduced CSF outflow to cervical lymph nodes. Superficial cervical lymphatics in aged mice had increased endothelial cell expression of Nos3, encoding endothelial nitric oxide synthase (eNOS), but had less eNOS protein and impaired nitric oxide signalling. Manipulation of superficial cervical lymphatics through intact skin by a force-regulated mechanical device doubled CSF outflow and corrected drainage impairment in aged mice. This manipulation increased CSF outflow by compressing superficial cervical lymphatics while having little effect on their normal spontaneous contractions. Overall, the findings highlight the importance of superficial cervical lymphatics for CSF outflow and the potential for reversing CSF drainage impairment by non-invasive mechanical stimulation.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures






References
-
- Key, A. & Retzius, G. Studien in der Anatomie des Nervensystems und des Bindegewebes: 1. Hälfte Vol. 1 (Samson & Wallin, 1875).
-
- Bradbury, M. W. B. & Cserr, H. F. Drainage of cerebral interstitial fluid and of cerebrospinal fluid into lymphatics. Exp. Biol. Lymph. Circ.9, 355–394 (1985).
-
- Cserr, H. F., Harling-Berg, C. J. & Knopf, P. M. Drainage of brain extracellular fluid into blood and deep cervical lymph and its immunological significance. Brain Pathol.2, 269–276 (1992). - PubMed
-
- Kida, S., Pantazis, A. & Weller, R. O. CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathol. Appl. Neurobiol.19, 480–488 (1993). - PubMed
-
- Zakharov, A., Papaiconomou, C., Djenic, J., Midha, R. & Johnston, M. Lymphatic cerebrospinal fluid absorption pathways in neonatal sheep revealed by subarachnoid injection of Microfil. Neuropathol. Appl. Neurobiol.29, 563–573 (2003). - PubMed

