Molecular gradients shape synaptic specificity of a visuomotor transformation
- PMID: 40468081
- PMCID: PMC12350164
- DOI: 10.1038/s41586-025-09037-4
Molecular gradients shape synaptic specificity of a visuomotor transformation
Abstract
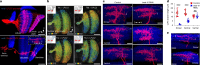
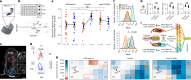
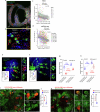
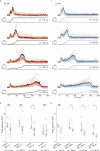
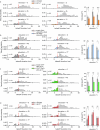
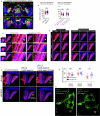
How does the brain convert visual input into specific motor actions1,2? In Drosophila, visual projection neurons (VPNs)3,4 perform this visuomotor transformation by converting retinal positional information into synapse number in the brain5. The molecular basis of this phenomenon remains unknown. We addressed this issue in LPLC2 (ref. 6), a VPN type that detects looming motion and preferentially drives escape behaviour to stimuli approaching from the dorsal visual field with progressively weaker responses ventrally. This correlates with a dorsoventral gradient of synaptic inputs into and outputs from LPLC2. Here we report that LPLC2 neurons sampling different regions of visual space exhibit graded expression of cell recognition molecules matching these synaptic gradients. Dpr13 shapes LPLC2 outputs by binding DIP-ε in premotor descending neurons mediating escape. Beat-VI shapes LPLC2 inputs by binding Side-II in upstream motion-detecting neurons. Gain-of-function and loss-of-function experiments show that these molecular gradients act instructively to determine synapse number. These patterns, in turn, fine-tune the perception of the stimulus and drive the behavioural response. Similar transcriptomic variation within neuronal types is observed in the vertebrate brain7 and may shape synapse number via gradients of cell recognition molecules acting through both genetically hard-wired programs and experience.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures










References
-
- Shin, S., Crapse, T. B., Angeles, L., Mayo, J. P. & Sommer, M. A. in Encyclopedia of Neuroscience (eds Binder, M. D., Hirokawa, N. & Windhorst, U.) 4354–4359 (Springer, 2009).
-
- Andersen, R. A., Snyder, L. H., Li, C. S. & Stricanne, B. Coordinate transformations in the representation of spatial information. Curr. Opin. Neurobiol.3, 171–176 (1993). - PubMed
-
- Otsuna, H. & Ito, K. Systematic analysis of the visual projection neurons of Drosophila melanogaster. I. Lobula-specific pathways. J. Comp. Neurol.497, 928–958 (2006). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

