Beyond the homozygous paradigm: symptomatic partial biotinidase deficiency in a heterozygous child-first case report from Nepal
- PMID: 40468249
- PMCID: PMC12135278
- DOI: 10.1186/s12887-025-05822-2
Beyond the homozygous paradigm: symptomatic partial biotinidase deficiency in a heterozygous child-first case report from Nepal
Abstract
Background: Biotinidase deficiency (BTD) is a rare genetic condition inherited in an autosomal recessive pattern that affects multiple systems. Biotinidase (EC 3.5.1.12) cleaves the vitamin, biotin, from the biocytin and the dietary protein-bound sources, and recycles the biotin. It manifests with a range of neurocutaneous symptoms, including seizures, hypotonia, ataxia, skin rashes, alopecia, hearing loss, optic atrophy, and metabolic crises that resemble sepsis, delay in identification and prompt treatment, results in irreversible brain damage, coma, and, in severe cases, death. Newborn screening can help in early diagnosis as it is amenable to treatment with pharmacological doses of biotin. There has been no study from Nepal about Biotinidase deficiency highlighting the clinical features, diagnosis and response to treatment.
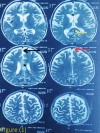
Case presentation: An 18-month-old male, born at term via normal delivery to non-consanguineous parents, with a normal neonatal period, presented to Kanti Children’s Hospital (KCH) with a 3-month history of multiple abnormal body movements and developmental regression. There were no known familial or genetic illnesses. Developmentally, he achieved milestones appropriately until 12 months of age, since then 15 months of age he had development regression. Ophthalmology study showed bilateral pallor of optic disc. MRI, EEG was done thinking of the inborn error of metabolism. A whole exome sequencing identified a heterozygous pathogenic variant in the BTD gene (c.38_44delinsTCC, p.Cys13Phefs*36), and retrospective enzyme assay confirmed partial biotinidase deficiency (3.20 nmol/min/mL; ~25% of mean normal activity). Prompt clinical improvement following biotin supplementation supported the diagnosis despite a non-specific initial presentation. He was then managed with oral biotin 10 mg and supportive care. Follow-up assessments at 3, 6, and 12 months post-treatment showed remarkable improvement.
Conclusions: This case highlights the clinical and diagnostic challenges of biotinidase deficiency (BTD) in resource-limited settings like Nepal, where the absence of newborn screening and limited molecular diagnostic capacity delay timely intervention. Increased clinician awareness, and local research to better understand the spectrum of BTD mutations, their clinical implications and to analyze the effectiveness of implementation of newborn screening programs. Early recognition and treatment of BTD can significantly improve patient outcomes and reduce the burden of this potentially devastating yet treatable disorder.
Clinical trial number: Not applicable.
Supplementary Information: The online version contains supplementary material available at 10.1186/s12887-025-05822-2.
Keywords: Biotin; Biotinidase; MCD; Newborn screening.
Conflict of interest statement
Declarations. Ethical approval: Case reports are exempt from ethical approval in our institution, Kanti Children’s Hospital, Maharajgunj, Kathmandu. Consent to participate and publication: Written informed consent was obtained from the patient’s parents for participation and publication of this case reports and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request. Competing interests: The authors declare no competing interests.
Figures
References
-
- Strovel ET, Cowan TM, Scott AI, Wolf B. Laboratory diagnosis of biotinidase deficiency, 2017 update: A technical standard and guideline of the American college of medical genetics and genomics. Genet Sci. 2017;19(10). - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

