The immunosuppressive effect of glucocorticoids in human primary T cells is mainly mediated via a rapid inhibition of the IL-2/IL-2R signaling axis
- PMID: 40468327
- PMCID: PMC12139085
- DOI: 10.1186/s12964-025-02266-0
The immunosuppressive effect of glucocorticoids in human primary T cells is mainly mediated via a rapid inhibition of the IL-2/IL-2R signaling axis
Abstract
Background: Glucocorticoids (GCs) are highly effective anti-inflammatory drugs that suppress T-cell activation, cytokine production, and T-cell proliferation. Nevertheless, at which molecular level and how fast GCs exert their immunosuppressive effect in T cells still remains elusive, as inconsistent genomic and non-genomic mechanisms of action have been proposed. One model postulates that GCs quickly inhibit proximal T-cell receptor (TCR) signaling via a non-genomic mechanism, whereas others have shown a strong inhibition of interleukin-2 (IL-2) transcription at later stages of T-cell activation. Due to their therapeutic significance, we have decided to shed light onto this issue and investigated how fast and at which level GCs inhibit T-cell activation by analyzing TCR and IL-2 signaling.
Methods: We utilized primary human T cells isolated from healthy donors, which were stimulated with immobilized CD3/CD28 antibodies. These cells were treated with three different GCs, diflorasone, dexamethasone, and prednisolone.
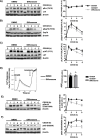
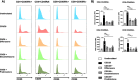
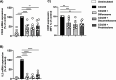
Results: Analyses of signaling kinetics revealed that GCs did not affect early TCR signaling as suggested by the normal phosphorylation levels of lymphocyte-specific protein tyrosine kinase (Lck), zeta-chain-associated protein kinase 70 (Zap70), linker for activation of T cells (LAT), and unchanged Ca2+ influx. Conversely, we found that GCs strongly and rapidly suppressed the activation of the Janus kinase (Jak)/ signal transducer and activator of transcription (STAT) pathway within 4-6 h upon CD3/CD28 stimulation in primary human T cells. This observation was in line with a strong inhibition of cytokine production and with the impaired upregulation of the IL-2 receptor (IL-2R) upon GC treatment, thus resulting in the abrogation of T-cell proliferation.
Conclusions: Our study, by showing that GCs rapidly suppress the IL-2/IL-2R expression and signaling without significantly affecting proximal TCR signaling, has highlighted a clear mechanism of action of GCs that contributes to their therapeutic efficacy.
Keywords: Erk1/2 activation; Glucocorticoids; IL-2 signaling; Immunosuppression; IκB activation; Jak/STAT pathway; T cells; TCR signaling.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The studies involving humans were approved by the Ethics Committee of Otto-von-Guericke University Magdeburg and the University Hospital of Magdeburg (Approval No. 175/18). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures







Similar articles
-
Inhibition of IL-2-induced Jak-STAT signaling by glucocorticoids.Proc Natl Acad Sci U S A. 2000 Aug 15;97(17):9573-8. doi: 10.1073/pnas.160099797. Proc Natl Acad Sci U S A. 2000. PMID: 10920190 Free PMC article.
-
Pretreatment with glucocorticoids enhances T-cell effector function: possible implication for immune rebound accompanying glucocorticoid withdrawal.Cell Transplant. 1999 Nov-Dec;8(6):637-47. doi: 10.1177/096368979900800610. Cell Transplant. 1999. PMID: 10701493
-
Enhanced interaction between SEC2 mutant and TCR Vβ induces MHC II-independent activation of T cells via PKCθ/NF-κB and IL-2R/STAT5 signaling pathways.J Biol Chem. 2018 Dec 21;293(51):19771-19784. doi: 10.1074/jbc.RA118.003668. Epub 2018 Oct 23. J Biol Chem. 2018. PMID: 30352872 Free PMC article.
-
Lymphocyte Activation in Health and Disease.Crit Rev Immunol. 2017;37(2-6):439-462. doi: 10.1615/CritRevImmunol.v37.i2-6.120. Crit Rev Immunol. 2017. PMID: 29773029 Review.
-
Glucocorticoid signaling: a nongenomic mechanism for T-cell immunosuppression.Trends Mol Med. 2007 Apr;13(4):158-63. doi: 10.1016/j.molmed.2007.02.001. Epub 2007 Feb 9. Trends Mol Med. 2007. PMID: 17293163 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

