Macrophages generated in 3D mechanical microenvironment contribute to recruiting Peritonitis-associated neutrophils in vivo
- PMID: 40468354
- PMCID: PMC12139310
- DOI: 10.1186/s13287-025-04413-3
Macrophages generated in 3D mechanical microenvironment contribute to recruiting Peritonitis-associated neutrophils in vivo
Abstract
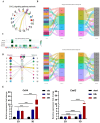
Backgroud: The influence of scaffolds with bone marrow (BM) niche-like mechanical properties on the stemness and lineage differentiation of hematopoietic stem cells (HSCs) in vitro has been studied. Previous research has demonstrated that 3D collagen hydrogels can regulate the differentiation trajectory of late myeloid progenitors, leading to the specialization of '3D-macrophages' that express various chemokine genes, including Cxcl2 and Cd14. Comprehensive transcriptional profiles in single-cell level have characterized the interaction between 3D-macrophages and neutrophil clusters through the CXCL2/CXCR2 ligand-receptor binding. Therefore, in this study, we aim to confirm the recruited effect of 3D-macrophage subsets on neutrophils during the immune process.
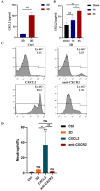
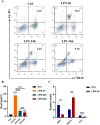
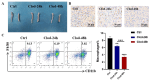
Methods: In this study, 3D collagen hydrogels were constructed to culture HSCs in vitro, then CD14+ cells, 3D-macrophages, which generated from HSCs regulated by mechanical microenvironment were purified from 3D gels by using specific antibody CD14 labeling and flow cytometry. First of all, the ability of 3D-macrophages to recruit neutrophils by secreting CXCL2 ligands was investigated in vitro by ELISA and Transwell experiments in vitro. Then, macrophage-deficient mice models were constructed by clodronate liposomes, after 3D-macrophages were transplanted into this models, lipopolysaccharide (LPS) was used to repeat peritonitis to investigate whether 3D-macrophages could recruit neutrophils by secreting CXCL2 ligands and maintain the output of HSCs pool to granulocyte-monocyte progenitor cells (GMPs) pool to sustain the subsequent immune response.
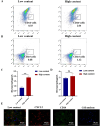
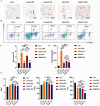
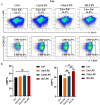
Results: In vitro experiments show that 3D-macrophages can recruit neutrophils by expressing Cxcl2. The effect of recruitment can also be observed in the peritonitis mice model, where macrophage ablation is experienced, followed by 3D-macrophage transplantation. CD14+ macrophages reach the site of inflammation, not only rescuing the immune deficiency caused by the absence of tissue macrophages but also maintaining the output of the common myeloid progenitor cells (CMPs) pool to the GMPs pool in the BM, thereby maintaining the production of mature immune cells during infection. The study revealed the immune function of macrophage subsets derived from a 3D mechanical microenvironment, which are expected to serve as a potential cell material for clinical significance.
Conclusion: These results explored the possible immune function of 3D-macrophages derived from 3D gels, assisting in comprehensively understand immune cell interaction in bone marrow hematopoietic microenvironment. Furthermore, 3D-macrophages are expected to serve as a potential cell therapy for immune deficiency caused by the functional deficiency of macrophages.
Keywords: 3D mechanical microenvironment; Hematopoietic stem cells; Macrophage differentiation; Neutrophil recruitment.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Experiments related to laboratory animals were performed after the approval by Lab Animal Ethics & Welfare committee of the Northwestern Polytechnical University. Project name: Macrophages generated in 3D mechanical microenvironment contribute to recruiting peritonitis-associated neutrophils in vivo. Approval no.202401125, date of approval:2024-2-28. Consent for publication: Not applicable. Competing interests: The authors declare no conflict of interests.
Figures

Similar articles
-
Type I Interferon, Induced by Adenovirus or Adenoviral Vector Infection, Regulates the Cytokine Response to Lipopolysaccharide in a Macrophage Type-Specific Manner.J Innate Immun. 2024;16(1):226-247. doi: 10.1159/000538282. Epub 2024 Mar 25. J Innate Immun. 2024. PMID: 38527452 Free PMC article.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Tumor-Associated Neutrophils Recruit Macrophages and T-Regulatory Cells to Promote Progression of Hepatocellular Carcinoma and Resistance to Sorafenib.Gastroenterology. 2016 Jun;150(7):1646-1658.e17. doi: 10.1053/j.gastro.2016.02.040. Epub 2016 Feb 26. Gastroenterology. 2016. PMID: 26924089
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
References
-
- Barriga F, Ramírez P, Wietstruck A, Rojas N. Hematopoietic stem cell transplantation: clinical use and perspectives. Biol Res. 2012;45(3):307–16. - PubMed
-
- Choi JS, Harley BA. The combined influence of substrate elasticity and ligand density on the viability and biophysical properties of hematopoietic stem and progenitor cells. Biomaterials. 2012;33(18):4460–8. - PubMed
MeSH terms
Substances
Grants and funding
- 12002285/Innovative Research Group Project of the National Natural Science Foundation of China
- 2020JZ-11/Natural Science Foundation of Shaanxi Province
- 2022JQ-059/Natural Science Foundation of Shaanxi Province
- 2024-KFQN-2/the Open Funds for Shaanxi Provincial Key Laboratory of Infection and Immune Diseases
LinkOut - more resources
Full Text Sources
Research Materials

