The many connections of UFMylation with Alzheimer's disease: a comprehensive review
- PMID: 40468360
- PMCID: PMC12139329
- DOI: 10.1186/s13024-025-00855-8
The many connections of UFMylation with Alzheimer's disease: a comprehensive review
Abstract
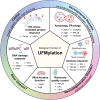
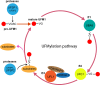
Alzheimer's disease (AD) is a complex neurodegenerative disorder that is characterized by the accumulation of pathologic tau and beta-amyloid proteins. UFMylation is an emerging ubiquitin-like post-translational modification that is crucial for healthy brain development. The UFM1 cascade was recently identified as a major modifier of tau aggregation in vitro and in vivo. Moreover, post-mortem AD brain shows pronounced alterations of UFMylation that are significantly associated with pathological tau, suggesting UFM1 might indeed be a modifier of human disease. However, the link between AD and UFMylation is yet to be fully explored. Interestingly, the UFMylation cascade is known to play important roles for several pathways that are known to be altered in AD, such as the DNA damage response, ER homeostasis, autophagy and the immune response. This review discusses the many connections between UFMylation with AD pathogenesis, emphasizing the role of UFMylation in these pathways and their abnormalities in AD. Understanding these connections is important to elucidate molecular mechanisms how UFM1 may impact AD and to uncover novel therapeutic strategies targeting UFMylation pathways for disease modification.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

