Inflammation alters myeloid cell and oligodendroglial iron-handling in multiple sclerosis
- PMID: 40468400
- PMCID: PMC12135419
- DOI: 10.1186/s40478-025-02020-0
Inflammation alters myeloid cell and oligodendroglial iron-handling in multiple sclerosis
Abstract
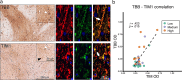
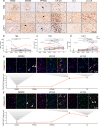
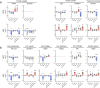
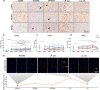
Changes in brain iron levels are a consistent feature of multiple sclerosis (MS) over its disease course. They encompass iron loss in oligodendrocytes in myelinated brain regions and iron accumulation in myeloid cells at so-called paramagnetic rims of chronic active lesions. Here, we explore the mechanisms behind this overall shift of iron from oligodendrocytes (OLs) to myeloid cells (MCs) and the loss of total brain-iron in MS. We investigated the expression of various iron importers and exporters, applying immunohistochemistry to a sample of control and MS autopsy cases. Additionally, we studied the transcriptional response of iron-related genes in primary rodent OL progenitor cells (OPCs) and microglia (MG) to various combinations of known MS-relevant pro-inflammatory stimuli together with iron loading. Histologically, we identified a correlation of OL-iron accumulation and the expression of the ferritin receptor TIM1 in myelinated white matter and observed an increase in the expression of iron-related proteins in myeloid cells at the lesion rims of MS plaques. qPCR revealed a marked increase of the heme scavenging and degradation machinery of MG under IFN-γ exposure, while OPCs changed to a more iron-inert phenotype with apparent decreased iron handling capabilities under MS-like inflammatory stimulation. Collectively, our data suggest that OL iron loss in MS is mainly due to a decrease in ferritin iron import. Iron accumulation in MCs at rims of chronic active lesions is in part driven by up-regulation of heme import and metabolism, while these cells also actively export ferritin.
Keywords: Iron loss; Iron rim lesions; Microglia; Multiple sclerosis; Oligodendrocytes.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: All studies on post-mortem human samples were performed in accordance with the Ethics Committee of the Medical University of Vienna (EC-vote: 1067/2024 and 1636/2019). Studies on primary rodent cell cultures were performed in accordance with the 2010/63/EU directive. As all procedures were performed post-sacrifice, no review board approval was needed. Animals were primarily sacrificed for diagnostics assays. Competing interests: The authors declare no competing interests.
Figures
References
-
- Calabrese M et al (2024) Determinants and biomarkers of progression independent of relapses in multiple sclerosis. Annals of Neurology - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

