Decoding diabetic kidney disease: a comprehensive review of interconnected pathways, molecular mediators, and therapeutic insights
- PMID: 40468401
- PMCID: PMC12139089
- DOI: 10.1186/s13098-025-01726-4
Decoding diabetic kidney disease: a comprehensive review of interconnected pathways, molecular mediators, and therapeutic insights
Abstract
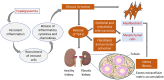
Background: Diabetic kidney disease (DKD) is a chronic kidney condition that arises from prolonged hyperglycaemia that can progress to kidney failure, severe morbidity, and mortality if left untreated. It is the major cause of chronic kidney disease among people who have diabetes, accounting for a significant percentage of patients with end-stage kidney disease who require kidney replacement therapy.
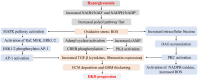
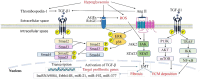
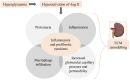
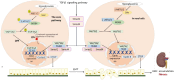
Main body: In DKD, numerous dysbalanced metabolic, haemodynamic, inflammatory signalling pathways, and molecular mediators interconnect, creating a feedback loop that promotes general kidney damage. Hyperglycaemia is the primary trigger for DKD and leads gradually to oxidative stress, inflammation, extracellular matrix deposition and fibrosis, glomerular hypertension, and intrarenal hypoxia. Key interconnected metabolic pathways are the hyperglycaemia-mediated polyol, hexosamine, protein kinase C, and advanced glycation end-products pathway hyperactivity. Concurrently, hyperglycaemia-induced renin-angiotensin-aldosterone system stimulation, alters the kidney intraglomerular haemodynamic leading to inflammation through Toll-like receptors, Janus kinase/signal transducer and activator of transcription, and nuclear factor-kappa B, transforming growth factor-beta-mediated excessive extracellular matrix accumulation and fibrosis. The resulting death signals trigger apoptosis and autophagy through Hippo, Notch, and Wnt/β-catenin pathway activation and microRNA dysregulation. These signals synergistically remodel the kidneys culminating in intrarenal hypoxia, podocyte dysfunction, hyperfiltration, epithelial-mesenchymal transition, and loss of kidney function. The resulting renal failure further upregulates these death pathways and mediators, giving rise to a vicious cycle that further worsens DKD.
Conclusion: This review provides an overview of the primary molecular mediators and signalling pathways leading to DKD; their interconnectivity at the onset and during DKD progression, the central role of transforming growth factor-beta via different pathways, the Hippo pathway kidney-specific response to hyperglycaemia, and how all mediators and transduction signals result in a vicious circle that exacerbates renal failure. The review gives therapeutic sights to these pathways as druggable targets for DKD management. Understanding these molecular events underlying the pathogenesis of DKD can bridge basic research and clinical application, facilitating the development of innovative management strategies.
Keywords: Chronic kidney disease; Diabetic nephropathy; End-stage kidney disease; Hippo signalling; Janus kinase/signal transducer and activator of transcription; Nuclear factor-kappa B; Renin–angiotensin–aldosterone system; Signal pathways; Toll-like receptors; Transforming growth factor-beta.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- Swaminathan SM, Rao IR, Bhojaraja MV, Attur RP, Nagri SK, Rangaswamy D, Shenoy SV, Nagaraju SP. Role of novel biomarker monocyte chemo-attractant protein-1 in early diagnosis & predicting progression of diabetic kidney disease: a comprehensive review. J Natl Med Assoc. 2024. 10.1016/j.jnma.2023.12.001. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

