A protocol for developing, disseminating, and implementing a core outcome set for caesarean scar ectopic pregnancy research: COSCAR
- PMID: 40468446
- PMCID: PMC12135297
- DOI: 10.1186/s13063-025-08879-7
A protocol for developing, disseminating, and implementing a core outcome set for caesarean scar ectopic pregnancy research: COSCAR
Abstract
Background: Caesarean scar ectopic pregnancy (CSEP) is the most common of type of uterine ectopic pregnancy and is associated with significant morbidity. Prompt diagnosis and treatment is therefore of paramount importance. Currently there is no universally agreed treatment option for CSEP supported by any national or international society. Studies evaluating CSEP management report many different outcomes and often define and measure success or complications of various treatments in different ways. This variation in reporting of outcomes leads to heterogeneity and an inability to directly, or reliably compare results of studies, leaving the question of what the optimal treatment is unanswered. We aim to develop a minimum set of outcomes that should be reported in all future research in CSEP.
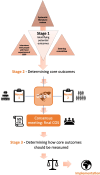
Methods: An international steering committee of key stakeholders, including researchers, healthcare professionals, patient advocates, and people with a lived experience of CSEP, has been established. A long list of potential outcomes will be identified from a systematic literature review and by interviewing people with a lived experience of CSEP. Key stakeholders will then be asked to prioritise the outcomes via a modified 2-round Delphi survey. Outcomes will be scored using a modified nine-point Likert scale that ranges from 1 (extremely unimportant) to 9 (extremely important) and an additional outcome of 'I can't rate the outcome because I don't know the outcome'. Finally, the steering group will refine by consensus the final core outcome set. The consensus process will result in a core outcome set that is internationally relevant to all key stakeholders. We will actively disseminate our findings to help improve clinical trials and guidelines with the ultimate aim of improving the diagnosis and management of CSEP.
Discussion: Implementing a core outcome set for CSEP will prevent research waste and improve patient centredness, by enabling reliable comparisons of different treatments for CSEP. This process will also help raise awareness of this condition, increasing clinician knowledge, which in turn will help them counsel patients more effectively, therefore benefiting professionals and patients alike. Expertise in diagnosing and managing this condition is currently focused in a handful of expert centres and many healthcare professionals are not always confident or comfortable in managing these patients and therefore refer them to other centres, which can be considerable distances from patients' localities. This core outcome set will aim to advance sharing of knowledge and spread expertise in time.
Trial registration: COMET 2903. Registered in November 2023. Available online on: https://www.comet-initiative.org/Studies/Details/2903 .
Keywords: Caesarean scar ectopic pregnancy; Core outcomes; Modified Delphi methodology; Treatment.
© 2025. Crown.
Conflict of interest statement
Declarations. Consent for publication: Not applicable. Competing interests: The authors declare they have no competing interests.
Figures
References
-
- Nijjar S, Jauniaux E, Jurkovic D. Definition and diagnosis of cesarean scar ectopic pregnancies. Best Pract Res Clin Obstet Gynaecol. 2023;89: 102360. - PubMed
-
- Nijjar S, Jauniaux E, Jurkovic D. Surgical evacuation of cesarean scar ectopic pregnancies. Best Pract Res Clin Obstet Gynaecol. 2023;89: 102361. - PubMed
-
- De Braud LV, Knez J, Mavrelos D, Thanatsis N, Jauniaux E, Jurkovic D. Risk prediction of major haemorrhage with surgical treatment of live cesarean scar pregnancies. Eur J Obstet Gynecol Reprod Biol. 2021;264:224–31. - PubMed
-
- Timor-Tritsch I, Buca D, Di Mascio D, Cali G, D’Amico A, Monteagudo A, et al. Outcome of cesarean scar pregnancy according to gestational age at diagnosis: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;258:53–9. - PubMed
-
- Timor-Tritsch IE. A cesarean scar pregnancy is not an ectopic pregnancy. Ultrasound Obstet Gynecol. 2022;59(4):424–7. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical