Overcoming temozolomide resistance in glioma: recent advances and mechanistic insights
- PMID: 40468460
- PMCID: PMC12139195
- DOI: 10.1186/s40478-025-02046-4
Overcoming temozolomide resistance in glioma: recent advances and mechanistic insights
Abstract
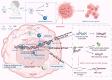
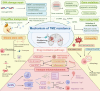
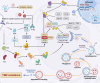
Temozolomide (TMZ) remains the cornerstone chemotherapy for glioma, yet intrinsic and acquired resistance mechanisms significantly limit its clinical effectiveness. This review summarizes the multifaceted molecular pathways contributing to TMZ resistance, including enhanced DNA repair mechanisms such as O6-methylguanine-DNA methyltransferase (MGMT), mismatch repair (MMR), and base excision repair (BER). Additional resistance factors include genetic mutations that affect the drug response, dysregulated non-coding RNAs (miRNAs, lncRNAs, and circRNAs), glioma stem cells (GSCs), cytoprotective autophagy, an immunosuppressive tumor microenvironment (TME), altered signaling pathways, and active drug efflux transporters. Recent advancements to overcome these resistance mechanisms, including enhancing TMZ bioavailability through nanoparticle-based delivery systems and the inhibition of efflux transporters, have been explored. Novel therapeutic approaches that target DNA repair pathways and manipulate autophagy are highlighted. Immunotherapeutic interventions reversing immune suppression and metabolic strategies targeting tumor metabolism offer additional avenues. Emerging therapies such as CRISPR-based gene editing, phytochemical combinations, repurposed drugs, and novel TMZ analogs designed to bypass MGMT-mediated resistance are also discussed. This review highlights current developments and identifies emerging areas, with the goals of enhancing clinical outcomes and prolonging survival for glioma patients.
Keywords: Chemoresistance; Glioma; Temozolomide; Treatment strategies.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


References
-
- Weller M et al (2024) Glioma. Nat Rev Dis Primers 10(1):33 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

