Feasibility and outcome of genomics-guided treatment selection in advanced cancer - the MEGALiT explorative clinical trial
- PMID: 40468525
- PMCID: PMC12160592
- DOI: 10.2340/1651-226X.2025.43366
Feasibility and outcome of genomics-guided treatment selection in advanced cancer - the MEGALiT explorative clinical trial
Abstract
Background: Precision cancer medicine (PCM) is key to advancing cancer treatment beyond the standard of care. We performed an explorative clinical trial, MEGALiT, to investigate the feasibility, safety, and clinical benefit of genomics-based PCM in advanced cancer.
Methods: MEGALiT recruited adult patients with advanced solid tumors refractory to standard treatment. Tumor DNA from newly acquired biopsies or ctDNA were analyzed for alterations targetable with the PD-L1 inhibitor atezolizumab, the MEK inhibitor cobimetinib, the mTOR inhibitor everolimus, or the PARP-inhibitor niraparib. Any other 'in study' treatment was left to the discretion of the physician.
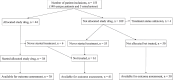
Results: Outcome data are reported for 153 patients. The median age was 65 years and the most common diagnoses were colorectal, prostate, and ovarian cancer. The median time from study inclusion to the Molecular Tumor Board was 35 days for tumor sampling by biopsy and 21 days by ctDNA. Of the 44 patients allocated to a study drug, 38 started treatment. The median follow-up was 1.9 years. Of the patients on a study drug and evaluable for tumor response, 6% (2/32) had partial remission, and 25% (8/32) had disease control at 16 weeks. Median overall survival for patients starting a study drug was longer, 7.4 months, compared to 2.7 months for the 61 untreated patients (HR 0.43; log-rank p < 0.0001), but shorter than for the 50 patients receiving treatment of physician's choice, 11.8 months (HR 0.55; log-rank p = 0.012). No significant procedure- or drug-related severe adverse events were observed.
Interpretation: Genomics-guided treatment selection in advanced cancer is feasible and safe. However, evidence of patient benefit warrants further investigation.
Conflict of interest statement
The authors report no competing interests related to the study.
Figures


Similar articles
-
Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): a multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial.Lancet Oncol. 2015 Oct;16(13):1324-34. doi: 10.1016/S1470-2045(15)00188-6. Epub 2015 Sep 3. Lancet Oncol. 2015. PMID: 26342236 Clinical Trial.
-
Genomic correlates of response and resistance to the irreversible FGFR1-4 inhibitor futibatinib based on biopsy and circulating tumor DNA profiling.Ann Oncol. 2025 Apr;36(4):414-425. doi: 10.1016/j.annonc.2024.11.017. Epub 2024 Dec 11. Ann Oncol. 2025. PMID: 39672383 Clinical Trial.
-
Serial monitoring of genomic alterations in circulating tumor cells of ER-positive/HER2-negative advanced breast cancer: feasibility of precision oncology biomarker detection.Mol Oncol. 2022 May;16(10):1969-1985. doi: 10.1002/1878-0261.13150. Epub 2021 Dec 20. Mol Oncol. 2022. PMID: 34866317 Free PMC article.
-
Association of Circulating Tumor DNA With Disease-Free Survival in Breast Cancer: A Systematic Review and Meta-analysis.JAMA Netw Open. 2020 Nov 2;3(11):e2026921. doi: 10.1001/jamanetworkopen.2020.26921. JAMA Netw Open. 2020. PMID: 33211112 Free PMC article.
-
Personalized Medicine: Genomics Trials in Oncology.Trans Am Clin Climatol Assoc. 2015;126:133-43. Trans Am Clin Climatol Assoc. 2015. PMID: 26330667 Free PMC article. Review.
Cited by
-
The promises of precision medicine - voices from the Nordics.Acta Oncol. 2025 Jun 11;64:775-777. doi: 10.2340/1651-226X.2025.43987. Acta Oncol. 2025. PMID: 40500958 Free PMC article. No abstract available.
References
-
- Mateo J, Chakravarty D, Dienstmann R, Jezdic S, Gonzalez-Perez A, Lopez-Bigas N, et al. A framework to rank genomic alterations as targets for cancer precision medicine: the ESMO Scale for Clinical Actionability of molecular Targets (ESCAT). Ann Oncol. 2018;29:1895–902. 10.1093/annonc/mdy263 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

