Aggregation, Photoluminescence, and Cytotoxicity of Pt(II) and Re(I) Complexes Bearing Multimodal-Coordinating Luminophores
- PMID: 40468680
- PMCID: PMC12188160
- DOI: 10.1002/chem.202404115
Aggregation, Photoluminescence, and Cytotoxicity of Pt(II) and Re(I) Complexes Bearing Multimodal-Coordinating Luminophores
Abstract
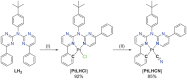
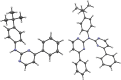
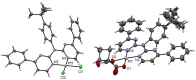
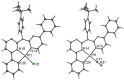
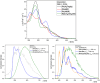
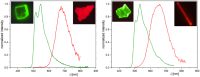
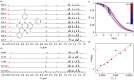
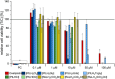
In this study, a polydentate ligand capable of adopting multiple coordination modes (N*N, C^N*N, and C^N*N^C) was established. Depending on the chelation conditions and the co-ligand used in the subsequent steps, nine complexes became accessible. The four Pt(II)-based species with N*N-coordination motif show tunable emission and cytotoxicity. Two Pt(II) complexes with C^N*N-type coordination were also obtained, and the exchange of the co-ligand from chlorido to cyanido led to an enhancement in the photoluminescence quantum yield from <0.02 to 0.55. These compounds exhibit distinct solid-state characteristics associated with Pt⋯Pt-interactions. Interestingly, the tetradentate C^N*N^C-coordination mode was not accessible, most likely due to kinetic reasons and insolubility-related purification hurdles. However, the use of a N*N-coordination mode for a Re(I) center yielded non-cytotoxic 3MLCT-state emitters. The multifaceted coordination modes available with the luminophoric ligand enable the design and realization of diverse metal complexes with tailored cytotoxicity and tendency towards intermolecular coupling, both in solution and in crystalline phases.
Keywords: Pt(II) complexes; Pt⋯Pt interactions; aggregation; cytotoxicity; photoluminescence.
© 2025 The Author(s). Chemistry – A European Journal published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








References
-
- Yersin H., Rausch A. F., Czerwieniec R., Hofbeck T., Fischer T., Coord. Chem. Rev. 2011, 255, 2622.
-
- Mao M., Peng J., Lam T.‐L., Ang W.‐H., Li H., Cheng G., Che C.‐M., J. Mater. Chem. C 2019, 7, 7230.
-
- Kalinowski J., Fattori V., Cocchi M., Williams J. G., Coord. Chem. Rev. 2011, 255, 2401.
-
- Lu W., Mi B.‐X., Chan M. C. W., Hui Z., Zhu N., Lee S.‐T., Che C.‐M. Chem. Commun. 2002, 2002, 206. - PubMed
-
- Tam A. Y.‐Y., Tsang D. P.‐K., Chan M.‐Y., Zhu N., Yam V. W.‐W., Chem. Commun. 2011, 47, 3383. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

