Association Between Baseline Neutrophil Count and Febrile Neutropenia Following Docetaxel and Ramucirumab With Prophylactic Pegfilgrastim
- PMID: 40468685
- PMCID: PMC12138040
- DOI: 10.1111/1759-7714.70099
Association Between Baseline Neutrophil Count and Febrile Neutropenia Following Docetaxel and Ramucirumab With Prophylactic Pegfilgrastim
Abstract
Background: Patients with non-small cell lung cancer (NSCLC) receiving docetaxel (DTX) and ramucirumab (RAM) regimen frequently experience febrile neutropenia (FN). We aimed to clarify the incidence rate and predictive factors of FN under prophylactic pegfilgrastim.
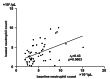
Methods: Fifty-four patients with NSCLC received DTX + RAM from 2018 to 2023 in our hospital. Age, gender, performance status (PS), treatment line, prior thoracic irradiation, body mass index (BMI), neutrophil count at baseline (BNC) and the lowest neutrophil count (LNC), serum albumin, and incidence of FN were recorded. The correlation between BNC and LNC was analyzed. We evaluated the association between BNC and FN using logistic regression analysis. The baseline characteristics of two groups stratified by the cutoff BNC using the ROC curve were compared.
Results: All the patients received prophylactic pegfilgrastim. Three (5.5%) patients experienced FN. There was a significant correlation between BNC and LNC (rs = 0.43, p = 0.0003). BNC and cardiovascular disease were significantly associated with FN (odds ratio 0.998, p = 0.0151 and odds ratio 28.64, p = 0.034). The receiver operating characteristic curve showed the cutoff value of BNC was 3000/μL (AUC 0.88). There was no significant difference in other baseline characteristics between the patients with BNC of 3000/μL or more and those with BNC less than 3000/μL.
Conclusion: Our data showed the incidence rate of FN receiving DTX + RAM with prophylactic pegfilgrastim was 5.5%. Despite prophylactic pegfilgrastim, BNC was correlated with LNC and might be predictive of FN. We should be careful of BNC even if it meets the criteria for starting DTX + RAM treatment.
Keywords: baseline neutrophil count; docetaxel; febrile neutropenia; pegfilgrastim; ramucirumab.
© 2025 The Author(s). Thoracic Cancer published by John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Garon E. B., Ciuleanu T.‐E., Arrieta O., et al., “Ramucirumab Plus Docetaxel Versus Placebo Plus Docetaxel for Second‐Line Treatment of Stage IV Non‐Small‐Cell Lung Cancer After Disease Progression on Platinum‐Based Therapy (REVEL): A Multicentre, Double‐Blind, Randomised Phase 3 Trial,” Lancet 384, no. 9944 (2014): 665–673. - PubMed
-
- Yoh K., Hosomi Y., Kasahara K., et al., “A Randomized, Double‐Blind, Phase II Study of Ramucirumab Plus Docetaxel vs Placebo Plus Docetaxel in Japanese Patients With Stage IV Non‐Small Cell Lung Cancer After Disease Progression on Platinum‐Based Therapy,” Lung Cancer 99, no. 99 (2016): 186–193. - PubMed
-
- Crawford J., Caserta C., and Roila F., “Hematopoietic Growth Factors: ESMO Clinical Practice Guidelines for the Applications,” Annals of Oncology 21 (2010): 248–251. - PubMed
-
- Masaoka T., “Evidence‐Based Recommendations for Antimicrobial Use in Febrile Neutropenia in Japan: Executive Summary,” Clinical Infectious Diseases 39, no. Supplement_1 (2004): S49–S52. - PubMed
-
- Klastersky J., “Febrile Neutropenia,” Current Opinion in Oncology 5, no. 4 (1993): 625–632. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

