MGBase: A Global, Observational Registry for Collaborative Research in Myasthenia Gravis
- PMID: 40468746
- PMCID: PMC12338016
- DOI: 10.1002/mus.28450
MGBase: A Global, Observational Registry for Collaborative Research in Myasthenia Gravis
Abstract
Introduction/aims: Patient registries are valuable tools for outcomes research in rare diseases such as myasthenia gravis (MG). Existing MG registries are limited by factors including a lack of geographical scope. MGBase has been designed as a global, observational registry aimed at studying clinical practice outcomes in MG.
Methods: MGBase was developed with the support of the independent MSBase Foundation. An international scientific leadership group (SLG) established a minimum dataset and outcome measures. Data are entered on a purpose-designed platform in real time and held in a web-based registry. Members can request access to the global dataset for investigator-driven substudies.
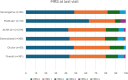
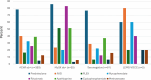
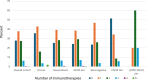
Results: MGBase data collection commenced in October 2021. From inception until April 2024, 565 patients from 16 clinics and 8 countries were enrolled. The cohort is 56% female, with a mean age of 57 (SD19) years at the last visit and a median disease duration of 5 (IQR 1.8, 10.8) years. Seventy-six percent of patients are acetylcholine receptor antibody positive (AChR ab+) and 7% have antibodies to muscle-specific kinase (MuSK ab+). At diagnosis, 33% of patients had ocular MG. Immunotherapy was used in 87% of patients. A minority of patients (7%) required three or more concurrent immunotherapies. Thymectomy was performed in 24% of patients.
Discussion: MGBase is a global registry for collaborative research in MG. Interim analysis of registry data shows disease characteristics similar to those previously published. As global enrollments increase, the registry will generate clinical practice evidence of treatment outcomes, safety, and disease prognostic markers.
Keywords: database; epidemiology; global; myasthenia gravis; registry.
© 2025 The Author(s). Muscle & Nerve published by Wiley Periodicals LLC.
Conflict of interest statement
K. Buzzard has received speaker's honoraria and/or education support from Biogen, Teva, Novartis, Genzyme‐Sanofi, Roche, Merck, Argenx, UCB, CSL and Alexion; has been a member of advisory boards for Merck, Alexion, Argenx, UCB and Biogen. She declares no competing interest regarding this manuscript.
A. Van der Walt has served on advisory boards and receives unrestricted research grants from Novartis, Biogen, Merck, and Roche. She has received speaker's honoraria and travel support from Novartis, Roche, and Merck. She receives grant support from the National Health and Medical Research Council of Australia and MS Research Australia.
H. Butzkueven has received institutional (Monash University) funding from Biogen, Roche, Merck, Alexion and Novartis; has carried out contracted research for Novartis, Merck, Roche and Biogen; has taken part in speakers' bureaus for Biogen, Novartis, Roche and Merck; has received personal compensation from Oxford Health Policy Forum for the Brain Health Steering Committee.
W. Zhang declares no conflicts of interest regarding this manuscript.
C. Barnett‐Tapia has been a member on the advisory board for Argenx, Alexion, Janssen, and UCB. Consultant for Argenx, Alexion, UCB and Janssen.
G. Cutter has been on the Data and Safety Monitoring Boards for Applied Therapeutics, AI therapeutics, AMO Pharma, Argenx, Astra‐Zeneca, Avexis Pharmaceuticals, Bristol Meyers Squibb/Celgene, CSL Behring, Cynata Therapeutics, DiameticaTherapeutics, Horizon Pharmaceuticals, Immunic, Inhibrix, Karuna Therapeutics, Kezar Life Sciences, Medtronic, Merck, Mitsubishi Tanabe Pharma Holdings, Prothena Biosciences, Novartis, Pipeline Therapeutics (Contineum), Regeneron, Sanofi‐Aventis, Reata Pharmaceuticals, Teva Pharmaceuticals, United BioSource LLC, University of Texas Southwestern, Visioneering Technologies Inc.
J. Heckmann has received honoraria for serving as speaker for Roche and for participating in meetings and advisory boards for Merck and Argenx. She declares no competing interest regarding this manuscript.
S. Reddel has received funds over the last 5 years including but not limited to travel support, honoraria, trial payments, research and clinical support to the institution has been received: Alexion, Biogen, Merck, Novartis, Roche.
Pamela Farr, Carolyn Tran, Charlotte Sartori, Dusko Stupar, Rein More, Linda Sim, Alison Le, and Qingxiao Tang are employees of the MSBase Foundation, the legal entity that operates the MGBase Registry.
Figures
References
-
- Kalincik T. and Butzkueven H., “The MSBase Registry: Informing Clinical Practice,” Multiple Sclerosis 25 (2019): 1828–1834. - PubMed
-
- Anil R., Kumar A., Alaparthi S., et al., “Exploring Outcomes and Characteristics of Myasthenia Gravis: Rationale, Aims and Design of Registry—The EXPLORE‐MG Registry,” Journal of the Neurological Sciences 414 (2020): 116830. - PubMed
-
- Ruiter A. M., Strijbos E., de Meel R., et al., “Accuracy of Patient‐Reported Data for an Online Patient Registry of Autoimmune Myasthenia Gravis and Lambert‐Eaton Myasthenic Syndrome,” Neuromuscular Disorders 31 (2021): 622–632. - PubMed
-
- Sipilä J. O. T., Soilu‐Hänninen M., Rautava P., and Kytö V., “Hospital Admission and Prevalence Trends of Adult Myasthenia Gravis in Finland in 2004‐2014: A Retrospective National Registry Study,” Journal of the Neurological Sciences 407 (2019): 116520. - PubMed
-
- Fulvio B. and Mantegazza R., “European Database for Myasthenia Gravis: A Model for an International Disease Registry,” Neurology 83 (2014): 189–191. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

