HCN2-Associated Neurodevelopmental Disorders: Data from Patients and Xenopus Cell Models
- PMID: 40468825
- PMCID: PMC12226820
- DOI: 10.1002/ana.27277
HCN2-Associated Neurodevelopmental Disorders: Data from Patients and Xenopus Cell Models
Abstract
Objective: We aimed to characterize the phenotypic spectrum and functional consequences associated with variants in HCN2, encoding for the hyperpolarization-activated cyclic nucleotide (HCN) gated channel 2.
Methods: GeneMatcher facilitated the recruitment of 21 individuals with HCN2 variants from 15 unrelated families, carrying HCN2 variants. In vitro functional studies were performed by electrophysiology with Xenopus laevis oocytes and membrane trafficking was investigated in HEK cells by confocal imaging. Structural 3D-analysis of the HCN2 variants was performed.
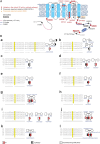
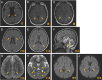
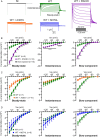
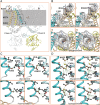
Results: The phenotypic spectrum included developmental delay/intellectual disability (DD/ID, 17/21), epilepsy (10/21), language disorders (16/21), movement disorders (12/21), and axial hypotonia (10/21). Thirteen pathogenic variants (12 new and 1 already described) were identified: 11 missense (8 monoallelic and 3 biallelic), 1 recurrent inframe deletion (monoallelic), and 1 frameshift (biallelic). Functional analysis of p.(Arg324His) variant showed a strong increase of HCN2 conductance, whereas p.(Ala363Val) and p.(Met374Leu) exhibited dominant negative effects. The p.(Leu377His), p.(Pro493Leu), and p.(Gly587Asp) variants rendered HCN2 electrophysiologically silent and impaired membrane trafficking. Structural 3D-analysis revealed that, except for p.(Arg324His), all variants altered HCN2 stability.
Interpretation: Our findings broadened the HCN2 disease clinical spectrum to include DD/ID with or without epilepsy. Functional analysis in cellular models reveal that pathogenic HCN2 variants can cause either loss-of-function or gain-of-function, providing critical information for the development of targeted therapies for HCN2-related disorders. ANN NEUROL 2025;98:573-589.
© 2025 The Author(s). Annals of Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures



References
-
- Reid CA, Berkovic SF, Petrou S. Mechanisms of human inherited epilepsies. Prog Neurobiol 2009;87:41–57. - PubMed
-
- Avanzini G, Franceschetti S, Mantegazza M. Epileptogenic channelopathies: experimental models of human pathologies. Epilepsia 2007;48:51–64. - PubMed
-
- Biel M, Wahl‐Schott C, Michalakis S, Zong X. Hyperpolarization‐activated cation channels: from genes to function. Physiol Rev 2009;89:847–885. - PubMed
-
- Poolos NP. Hyperpolarization‐activated cyclic nucleotide‐gated (HCN) ion channelopathy in epilepsy [Internet]. In: Noebels J, Avoli M, Rogawski M, et al., eds. Jasper's basic mechanisms of the epilepsies. Oxford: Oxford University Press, 2012:85‐96. Available from: 10.1093/med/9780199746545.001.0001/med-9780199746545-chapter-7. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

