Incidence of AIDS-defining Conditions among Adults with Perinatally Acquired HIV after Transition to Adult HIV Care in the United States and Canada, 2000-2022
- PMID: 40469005
- PMCID: PMC12375960
- DOI: 10.1093/cid/ciaf300
Incidence of AIDS-defining Conditions among Adults with Perinatally Acquired HIV after Transition to Adult HIV Care in the United States and Canada, 2000-2022
Abstract
Background: Little is known about the incidence of AIDS-defining conditions (ADCs) among people with perinatally acquired HIV (PHIV) who transitioned to adult HIV care in the United States and Canada. We described the incidence among PHIV and compared it with non-PHIV and across calendar era.
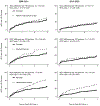
Methods: Using data from the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) from 2000-2022, we estimated weighted mean cumulative counts (MCC) of ADCs comparing people with PHIV and non-PHIV acquisition risk groups aged 18-40 engaged in adult HIV care. The weights accounted for differences in characteristics among HIV acquisition risk groups as well as informative censoring. We calculated 95% confidence intervals (CI) using bootstrapping. We stratified results before and after 2012, when immediate start of ART was recommended.
Results: There were 5,429 ADCs among 22,950 people with HIV. Among people with PHIV, the MCC of ADCs by three years in adult HIV care was 26 per 100 persons (95% CI: 15, 40) in 2000-2011 and 16 per 100 persons (95% CI: 5, 21) in 2012-2022. Within each calendar era, weighted MCCs of ADCs among people with PHIV were similar or lower than non-PHIV groups. Within each HIV acquisition risk group, weighted MCCs of ADCs were lower between 2000-2011 vs. 2012-2022.
Conclusion: People with PHIV who transitioned to adult HIV care did not experience a greater ADC incidence than people with non-PHIV. This emphasizes the importance of continued engagement in adult HIV care, as it provides critical opportunities for ADC prevention and management.
Keywords: AIDS defining conditions; HIV; lifetime survivors; perinatally acquired.
© The Author(s) 2025. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
C. R. L., D. K. N., T. R. S., J. L., A. A., and K. A. have received research grants from the NIH to their respective institutions. K. G. has received royalties from UpToDate; has served on a scientific advisory board for Shionogi and Pfizer (noncompensated); has received personal consulting fees from Spark HealthCare, Premier HealthCare, Harrison Consulting, and MedEd Learning; and has served on scientific advisory boards of Shionogi and Pfizer. A. A. has previously served on the scientific advisory boards of Gilead and Merck and as a site investigator under a clinical research contract with Gilead managed through Johns Hopkins University. K. A. has received royalties from Coursera. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources

