Postnatal hyperosmolality alters development of hypothalamic feeding circuits with context-specific changes in ingestive behavior
- PMID: 40469114
- PMCID: PMC12136832
- DOI: 10.1016/j.isci.2025.112284
Postnatal hyperosmolality alters development of hypothalamic feeding circuits with context-specific changes in ingestive behavior
Abstract
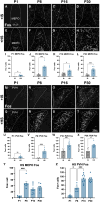
Drinking and feeding are tightly coordinated homeostatic events and the paraventricular nucleus of the hypothalamus (PVH) represents a possible node of neural integration for signals related to energy and fluid homeostasis. We used TRAP2;Ai14 mice and Fos labeling to visualize neurons in the PVH and median preoptic nucleus (MEPO) responding to both water deprivation and feeding signals. We determined that structural and functional development of dehydration-sensitive inputs to the PVH precedes those of agouti-related peptide (AgRP) neurons, which convey hunger signals and are known to be developmentally programmed by nutrition. Moreover, we found that osmotic hyperstimulation of neonatal mice led to enhanced AgRP inputs to the PVH in adulthood, as well as disruptions to ingestive behaviors during high-fat diet feeding and dehydration-anorexia. Thus, development of feeding circuits is impacted not only by nutritional signals, but also by early perturbations to fluid homeostasis with context-specific consequences for coordination of ingestive behavior.
Keywords: Developmental biology; Developmental neuroscience; Neuroscience.
© 2025 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Update of
-
Early perturbations to fluid homeostasis alter development of hypothalamic feeding circuits with context-specific changes in ingestive behavior.bioRxiv [Preprint]. 2024 Oct 26:2024.10.25.620307. doi: 10.1101/2024.10.25.620307. bioRxiv. 2024. Update in: iScience. 2025 Mar 24;28(5):112284. doi: 10.1016/j.isci.2025.112284. PMID: 39484367 Free PMC article. Updated. Preprint.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

