Dissecting the dual role of OTU family proteins in tumor progression and immune escape
- PMID: 40469292
- PMCID: PMC12133852
- DOI: 10.3389/fimmu.2025.1544341
Dissecting the dual role of OTU family proteins in tumor progression and immune escape
Abstract
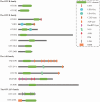
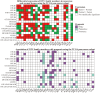
As a core mechanism regulating intracellular protein homeostasis, the dynamic equilibrium between ubiquitination and deubiquitination profoundly impacts the functionality and fate of target proteins. The Ovarian tumor domain (OTU) family, a vital subclass of deubiquitinating enzymes, comprises 16 members that mediate ubiquitin binding and hydrolysis through their characteristic OTU domain. Recent years have witnessed growing interest in OTU family members in oncology and immunology research. This review comprehensively elucidates the core mechanisms by which OTU members regulate tumor-associated signaling networks via substrate-specific deubiquitination. On one hand, they directly govern tumor cell proliferation, metastasis, and apoptosis by modulating the stability of key substrates. On the other hand, they orchestrate tumor progression through dynamic regulation of inflammatory intensity, immune response duration, and immune evasion mechanisms within the tumor microenvironment (TME), thereby constructing a multidimensional regulatory network in tumor development. These findings not only unveil the pivotal role of OTU family members in tumorigenesis and immune modulation but also establish a theoretical foundation for developing novel anti-tumor therapeutics targeting deubiquitination processes. Notably, OTUs emerge as high-potential therapeutic targets with high translational relevance for refining precision-guided tumor-immunotherapy integration strategies.
Keywords: OTU family; deubiquitinating enzymes; immune regulation; tumorigenesis; ubiquitination-deubiquitination balance.
Copyright © 2025 Tang, Li and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

