Ferroptosis in idiopathic pulmonary fibrosis: mechanisms, impact, and therapeutic opportunities
- PMID: 40469306
- PMCID: PMC12133551
- DOI: 10.3389/fimmu.2025.1567994
Ferroptosis in idiopathic pulmonary fibrosis: mechanisms, impact, and therapeutic opportunities
Abstract
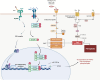
Idiopathic pulmonary fibrosis (IPF) is a fatal interstitial lung disease characterized by progressive scarring, alveolar destruction, and limited therapeutic options. Although the exact etiology of IPF remains unclear, emerging evidence suggests that ferroptosis, an iron-dependent form of regulated cell death driven by lipid peroxidation and oxidative stress, plays a significant role in its pathogenesis. Ferroptotic stress not only compromises alveolar epithelial cell integrity, but also triggers inflammatory responses and profibrotic signaling cascades that activate and sustain fibroblast dysfunction. This review delineates the core regulatory pathways of ferroptosis, iron metabolism, lipid peroxidation, antioxidant defenses, mitochondrial remodeling, and RNA editing, with an emphasis on their relevance in IPF. We explore how epithelial injury and macrophage-derived signals initiate ferroptosis, and how fibroblast subsets, shaped by scRNA-seq-defined heterogeneity and plasticity, respond to these cues by reinforcing ECM deposition and oxidative stress. Therapeutic avenues targeting ferroptosis, including antioxidant supplementation, iron chelation, and modulation of lipid metabolism, are discussed alongside cell-specific interventions and nanodelivery strategies. By integrating recent advances in molecular profiling and ferroptosis biology, this review provides a framework for leveraging ferroptosis as a tractable target in IPF and identifies novel directions for precision antifibrotic therapy.
Keywords: ferroptosis; idiopathic pulmonary fibrosis (IPF); immune homeostasis; interleukin; toll-like receptor; transforming growth factor; tumor necrosis factor; type I interferon.
Copyright © 2025 Yao, Liu, Zhao, Song, Huang and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Iron-laden macrophage-mediated paracrine profibrotic signaling induces lung fibroblast activation.Am J Physiol Cell Physiol. 2024 Oct 1;327(4):C979-C993. doi: 10.1152/ajpcell.00675.2023. Epub 2024 Aug 26. Am J Physiol Cell Physiol. 2024. PMID: 39183565
-
Ferroptosis: the potential key roles in idiopathic pulmonary fibrosis.Eur J Med Res. 2025 Apr 28;30(1):341. doi: 10.1186/s40001-025-02623-2. Eur J Med Res. 2025. PMID: 40296070 Free PMC article. Review.
-
Ferroptosis in organ fibrosis: Mechanisms and therapeutic approaches.Int Immunopharmacol. 2025 Apr 4;151:114341. doi: 10.1016/j.intimp.2025.114341. Epub 2025 Mar 1. Int Immunopharmacol. 2025. PMID: 40024213 Review.
-
Lipid metabolism in ferroptosis: mechanistic insights and therapeutic potential.Front Immunol. 2025 Mar 11;16:1545339. doi: 10.3389/fimmu.2025.1545339. eCollection 2025. Front Immunol. 2025. PMID: 40134420 Free PMC article. Review.
-
Calpain-1 Up-Regulation Promotes Bleomycin-Induced Pulmonary Fibrosis by Activating Ferroptosis.Am J Pathol. 2024 Dec;194(12):2272-2289. doi: 10.1016/j.ajpath.2024.09.004. Epub 2024 Sep 24. Am J Pathol. 2024. PMID: 39326733
References
-
- Lancaster L, Crestani B, Hernandez P, Inoue Y, Wachtlin D, Loaiza L, et al. . Safety and survival data in patients with idiopathic pulmonary fibrosis treated with nintedanib: pooled data from six clinical trials. BMJ Open Respir Res. (2019) 6:e000397. doi: 10.1136/bmjresp-2018-000397 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

