Dose-dependent effects of eltrombopag iron chelation on platelet formation
- PMID: 40469415
- PMCID: PMC12131944
- DOI: 10.1016/j.bvth.2025.100060
Dose-dependent effects of eltrombopag iron chelation on platelet formation
Abstract
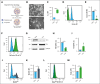
Iron deficiency is associated with thrombocytosis in patients, although thrombocytopenia can occur in cases of severe iron deficiency anemia. Eltrombopag (EP), a thrombopoietic agent approved for immune thrombocytopenia, also acts as an iron chelator. Our study demonstrates that megakaryocytes (MKs) exhibit an increased requirement for iron as they mature and acquire the ability to form proplatelets and release platelets. Although low EP concentrations maintain MK functions, high EP concentrations disrupt iron homeostasis, reducing proplatelet formation. Mechanistically, EP-dependent iron chelation impairs MK cytoskeletal dynamics, induces higher extracellular signal-regulated kinase 1/2 (ERK1/2) signaling, and reduces posttranslational glutathionylation of tubulin protein. Addition of exogenous iron or oxidized glutathione to high-dose EP-treated MKs counteracts the negative effect on iron status and ERK1/2 signaling, thereby rescuing proplatelet formation. Overall, these data reveal the complex role of iron status on MK cytoskeletal dynamics and platelet biogenesis and may explain the varied clinical manifestations of iron deficiency on platelet counts.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosures: The authors declare no competing financial interests.
Figures


References
-
- Evstatiev R, Gasche C. Iron sensing and signalling. Gut. 2012;61(6):933–952. - PubMed
-
- Cunha V, Ferreira M, Barosa R, Fonseca AG, Delerue F, Carvalho C. Iron-induced thrombocytopenia in severe iron-deficiency anemia. Expert Rev Hematol. 2015;8(2):247–251. - PubMed
-
- Holbro A, Volken T, Buser A, et al. Iron deficiency and thrombocytosis. Vox Sang. 2017;112(1):87–92. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
